Page 428 - IJB-10-5
P. 428
International Journal of Bioprinting Stability of 3D-printed PEO tablets
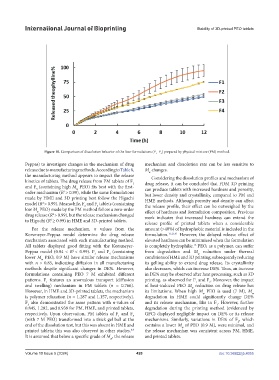
Figure 10. Comparison of dissolution behavior of the four formulations (F –F ) prepared by physical mixture (PM) method.
1 4
Peppas) to investigate changes in the mechanism of drug mechanism and dissolution rate can be less sensitive to
release due to manufacturing methods. According to Table 8, M changes.
w
the manufacturing method appears to impact the release Considering the dissolution profiles and mechanism of
kinetics of tablets. The drug release from PM tablets of F drug release, it can be concluded that FDM 3D printing
1
and F (containing high M PEO) fits best with the first- can produce tablets with increased hardness and porosity,
w
3
order mechanism (R > 0.99), while the same formulations but lower density and crystallinity, compared to PM and
2
made by HME and 3D printing best follow the Higuchi HME methods. Although porosity and density can affect
model (R > 0.99). Meanwhile, F and F tablets (containing the release profile, their effect can be outweighed by the
2
2
4
low M PEO) made by the PM method follow a zero-order effect of hardness and formulation composition. Previous
w
drug release (R > 0.99), but the release mechanism changed work indicates that increased hardness can extend the
2
to Higuchi (R ≥ 0.99) in HME and 3D-printed tablets.
2
release profile of printed tablets when a considerable
For the release mechanism, n values from the amount (>40%) of hydrophobic material is included in the
Korsmeyer-Peppas model determine the drug release formulation. 16,26,41 However, the delayed release effect of
mechanism associated with each manufacturing method. elevated hardness can be minimized when the formulation
All tablets displayed good fitting with the Korsmeyer- is completely hydrophilic. PEO, as a polymer, can suffer
16
Peppas model (0.84 < R < 0.99). F and F (containing from degradation and M reduction under thermal
2
2
w
4
lower M PEO, 0.9 M) have similar release mechanisms conditions of HME and 3D printing, subsequently reducing
w
with n > 0.85, indicating diffusion in all manufacturing its gelling ability to extend drug release. Its crystallinity
methods despite significant changes in DE%. However, also decreases, which can increase DE%. Thus, an increase
formulations containing PEO 7 M exhibited different in DE% may be observed after heat processing, such as 3D
patterns. F features an anomalous transport (diffusion printing, as observed for F and F . Moreover, the impact
1
2
1
and swelling) mechanism in PM tablets (n = 0.766). of heat-induced PEO M reduction on drug release has
w
However, in HME and 3D-printed tablets, the mechanism its limitations. When high M PEO is used (7 M), M
w
w
is polymer relaxation (n = 1.287 and 1.157, respectively). degradation in HME could significantly change DE%
F also demonstrated the same pattern with n-values of and its release mechanism, like in F . However, further
1
3
0.845, 1.282, and 0.939 for PM, HME, and printed tablets, degradation during the printing method (evidenced by
respectively. Upon observation, PM tablets of F and F GPC) displayed negligible impact on DE% or its release
1
3
(with 7 M PEO) transformed into a thick gel ball at the mechanisms. Similarly, variations in DE% of F , which
2
end of the dissolution test, but this was absent in HME and contains a lower M of PEO (0.9 M), were minimal, and
w
printed tablets; this was also observed in other studies. the release mechanism was consistent across PM, HME,
8,9
It is assumed that below a specific grade of M , the release and printed tablets.
w
Volume 10 Issue 5 (2024) 420 doi: 10.36922/ijb.4055