Page 426 - IJB-10-5
P. 426
International Journal of Bioprinting Stability of 3D-printed PEO tablets
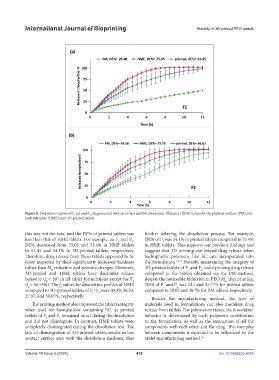
Figure 8. Dissolution curves of F (a) and F (b), presented with error bars and the dissolution efficiency (DE%) values for the physical mixture (PM), hot-
2
1
melt extrudate (HME), and 3D-printed tablets.
this was not the case, and the DE% of printed tablets was further delaying the dissolution process. For example,
less than that of HME tablets. For example, in F and F , DE% of F was 24.1% in printed tablets compared to 53.4%
1
3
3
DE% decreased from 75.05 and 53.4% in HME tablets in HME tablets. This supports our previous findings and
to 61.45 and 24.1% in 3D-printed tablets, respectively. suggests that 3D printing can extend drug release when
Therefore, drug release from these tablets appeared to be hydrophobic polymers, like EC, are incorporated into
more impacted by their significantly increased hardness the formulation. 16,26 Notably, maintaining the integrity of
rather than M reduction and porosity changes. Moreover, 3D-printed tablets of F and F could prolong drug release
w
4
3
3D-printed and HME tablets have dissimilar release compared to the tablets obtained via the PM method,
behavior (f < 50) in all tablet formulations except for F despite the noticeable reduction in PEO M after printing.
2
2
w
(f = 56.53%). The f values for dissolution profiles of HME DE% of F and F was 24.1 and 25.77% for printed tablets
2
4
2
3
compared to 3D-printed tablets of F –F were 43.89, 56.53, compared to 28.05 and 38.7% for PM tablets, respectively.
1
4
27.97, and 30.05%, respectively.
Besides the manufacturing method, the type of
The printing method also improved the tablet’s integrity materials used in formulations can also modulate drug
when used for formulations containing EC, as printed release from tablets. For polymer mixtures, the dissolution
tablets of F and F remained intact during the dissolution behavior is determined by each polymer’s contribution
3
4
and did not disintegrate. In contrast, HME tablets were to the formulation, as well as the interaction of all the
completely disintegrated during the dissolution test. The components with each other and the drug. This interplay
lack of disintegration of 3D-printed tablets results in low between components is expected to be influenced by the
contact surface area with the dissolution medium, thus tablet manufacturing method. 16
Volume 10 Issue 5 (2024) 418 doi: 10.36922/ijb.4055