Page 421 - IJB-10-5
P. 421
International Journal of Bioprinting Stability of 3D-printed PEO tablets
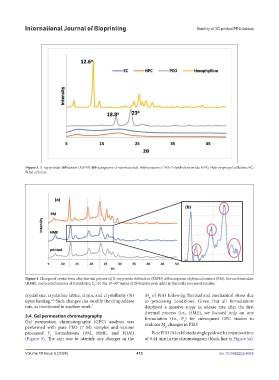
Figure 3. X-ray powder diffraction (XRPD) diffractograms of raw materials. Abbreviations: PEO: Polyethylene oxide; HPC: Hydroxypropyl cellulose; EC:
Ethyl cellulose.
Figure 4. Changes of crystal form after thermal process (a) X-ray powder diffraction (XRPD) diffractograms of physical mixture (PM), hot-melt extrudate
(HME), and printed samples of formulation F . (b) The 10–30° region of 2θ displays peak splits in thermally processed samples.
2
crystal size, crystalline lattice, strain, and crystallinity (%) M of PEO following thermal and mechanical stress due
w
upon heating. Such changes can modify the drug release to processing conditions. Given that all formulations
3,6
rate, as mentioned in another work. 7 displayed a massive surge in release rate after the first
thermal process (i.e., HME), we focused only on one
3.4. Gel permeation chromatography formulation (i.e., F ) for subsequent GPC studies to
Gel permeation chromatography (GPC) analysis was evaluate M changes in PEO.
1
performed with pure PEO (7 M) samples and various w
processed F formulations (PM, HME, and FDM) Pure PEO 7 M exhibited a single peak with a retention time
1
(Figure 5). The aim was to identify any changes in the of 9.44 min in the chromatogram (black line in Figure 5a).
Volume 10 Issue 5 (2024) 413 doi: 10.36922/ijb.4055