Page 75 - IJB-7-1
P. 75
Jing, et al.
A B
Figure 4. Tensile test of printed scaffolds (A) Stress-strain curve, (B) Enlarged view of initial range.
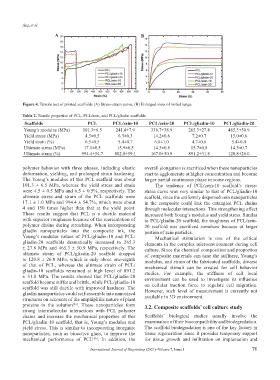
Table 2. Tensile properties of PCL, PCL/zein, and PCL/gliadin scaffolds
Scaffolds PCL PCL/zein-10 PCL/zein-20 PCL/gliadin-10 PCL/gliadin-20
Young’s modulus (MPa) 101.3±6.5 241.4±7.9 338.7±38.9 265.3±27.8 465.3±50.9
Yield stress (MPa) 4.5±0.5 6.3±0.3 14.2±0.6 7.2±0.7 15.0±0.6
Yield strain (%) 6.5±0.5 5.4±0.7 6.0±1.0 4.7±0.6 5.4±0.4
Ultimate stress (MPa) 17.1±0.5 15.9±0.5 14.3±0.8 15.7±0.8 14.5±0.7
Ultimate strain (%) 994.4±54.7 802.8±59.1 167.0±50.9 891.2±31.8 120.8±26.0
polymer behavior with three phases, including elastic overall elongation is sacrificed when these nanoparticles
deformation, yielding, and prolonged strain hardening. start to agglomerate at higher concentration and become
The Young’s modulus of this PCL scaffold was about larger partial continuous phase in some regions.
101.3 ± 6.5 MPa, whereas the yield stress and strain The tendency of PCL/zein-10 scaffold’s stress-
were 4.5 ± 0.5 MPa and 6.5 ± 0.5%, respectively. The strain curve was very similar to that of PCL/gliadin-10
ultimate stress and strain of the PCL scaffolds were scaffold, since the uniformly dispersed zein nanoparticles
17.1 ± 1.0 MPa and 994.4 ± 54.7%, which were about in the composite could link the entangled PCL chains
4 and 150 times higher than that at the yield point. through molecular interactions. This strengthening effect
These results suggest that PCL is a ductile material increased both Young’s modulus and yield stress. Similar
with superior roughness because of the reorientation of to PCL/gliadin-20 scaffold, the toughness of PCL/zein-
polymer chains during stretching. When incorporating 20 scaffold was sacrificed somehow because of larger
gliadin nanoparticles into the composite ink, the portion of zein particles.
Young’s modulus values of PCL/gliadin-10 and PCL/ Mechanical stimulation is one of the critical
gliadin-20 scaffolds dramatically increased to 265.3 elements in the complex microenvironment during cell
± 27.8 MPa and 465.3 ± 50.9 MPa, respectively. The culture. Since the chemical composition and proportion
ultimate strain of PCL/gliadin-20 scaffold dropped of composite materials can tune the stiffness, Young’s
to 120.8 ± 26.0 MPa, which is only about one-eighth modulus, and strain of the fabricated scaffolds, diverse
of that of PCL, whereas the ultimate strain of PCL/ mechanical stimuli can be created for cell behavior
gliadin-10 scaffolds remained at high level of 891.2 studies. For example, the stiffness of cell local
± 31.8 MPa. The results showed that PCL/gliadin-20 environment can be used to investigate its influence
scaffold became stiffer and brittle, while PCL/gliadin-10 on cellular traction force to regulate cell migration.
scaffold was still ductile with improved hardness. The
gliadin nanoparticles could self-assemble into nanosized However, such level of measurement is currently not
structures on accounts of the amphiphilic nature of plant available in 3D environment.
proteins in the solution [21] . These nanoparticles form 3.2. Composite scaffolds’ cell culture study
strong intermolecular interactions with PCL polymer
chains and increase the mechanical properties of this Scaffolds’ biological studies usually involve the
PCL/gliadin-10 scaffold, that is, Young’s modulus and examination of their biocompatibility and biodegradation.
yield stress. This is similar to incorporating inorganic The scaffold biodegradation is one of the key factors in
nanoparticles, such as bioactive glass, to improve the tissue regeneration since it provides temporary support
mechanical performance of PCL [26] . In addition, the for tissue growth and infiltration on implantation and
International Journal of Bioprinting (2021)–Volume 7, Issue 1 71