Page 90 - IJB-7-1
P. 90
Biodegradation, Antibacterial Performance and Cytocompatibility of SMLed ZK30-Cu-Mn
A B C D E
F G H I J
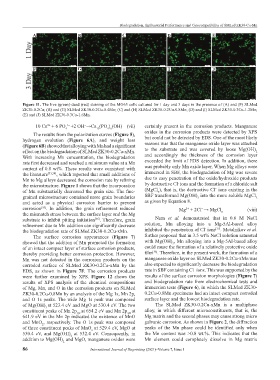
Figure 11. The live (green)-dead (red) staining of the MG63 cells cultured for 1 day and 3 days in the presence of (A) and (F) SLMed
ZK30-0.2Cu; (B) and (G) SLMed ZK30-0.2Cu-0.4Mn; (C) and (H) SLMed ZK30-0.2Cu-0.8Mn; (D) and (I) SLMed ZK30-0.3Cu-1.2Mn;
(E) and (J) SLMed ZK30-0.3Cu-1.6Mn.
10 Ca + 6 PO 3− +2 OH →Ca (PO ) (OH) (vii) certainly present in the corrosion products. Manganese
−
2+
4 10 4 6
The results from the polarization curves (Figure 5), oxides in the corrosion products were detected by XPS
hydrogen evolution (Figure 6A), and weight loss but could not be detected by EDS. One of the most likely
(Figure 6B) showed that alloying with Mn had a significant reasons was that the manganese oxide layer was attached
effect on the biodegradation of SLMed ZK30-0.2Cu-xMn. to the substrate and was covered by loose Mg(OH)
2
With increasing Mn concentration, the biodegradation and accordingly the thickness of the corrosion layer
rate first decreased and reached a minimum value at a Mn exceeded the limit of EDS detection. In addition, there
content of 0.8 wt%. These results were consistent with was probably only Mn oxide layer. When Mg alloys were
the literature [33,34] , which reported that small additions of immersed in SBF, the biodegradation of Mg was severe
Mn to Mg alloys decreased the corrosion rate by refining due to easy penetration of the oxide/hydroxide products
-
the microstructure. Figure 1 shows that the incorporation by destructive Cl ions and the formation of a chloride salt
−
of Mn substantially decreased the grain size. The fine- (MgCl ), that is, the destructive Cl ions existing in the
2
grained microstructure contained more grain boundaries SBF transformed Mg(OH) into the more soluble MgCl
2
2
and acted as a physical corrosion barrier to prevent as given by Equation 8.
corrosion . In addition, the grain refinement reduced Mg + 2Cl → MgCl (viii)
[35]
−
2+
the mismatch stress between the surface layer and the Mg 2
substrate to inhibit pitting initiation . Therefore, grain Nam et al. demonstrated that in 0.6 M NaCl
[36]
refinement due to Mn addition can significantly decrease solution, Mn alloying into a Mg-5Al-based alloy
-
[37]
the biodegradation rate of SLMed ZK30-0.2Cu-xMn. inhibited the penetration of Cl ions . Metalnikov et al.
The surface corrosion appearances (Figure 7) further proposed that in 3.5 wt% NaCl solution saturated
showed that the addition of Mn promoted the formation with Mg(OH) , Mn alloying into a Mg-5Al-based alloy
2
of an intact compact layer of surface corrosion products, could cause the formation of a relatively protective oxide
[38]
thereby providing better corrosion protection. However, film . Therefore, in the present work, the formation of a
Mn was not detected in the corrosion products on the manganese oxide layer on SLMed ZK30-0.2Cu-xMn was
corroded surface of SLMed ZK30-0.2Cu-xMn by the also expected to significantly decrease the biodegradation
−
EDS, as shown in Figure 7F. The corrosion products rate in SBF containing Cl ions. This was supported by the
were further examined by XPS. Figure 12 shows the results of the surface corrosion morphologies (Figure 7)
results of XPS analysis of the chemical compositions and biodegradation rate from electrochemical tests and
of Mg, Mn, and O in the corrosion products on SLMed immersion tests (Figure 6), in which the SLMed ZK30-
ZK30-0.2Cu-0.8Mn by an analysis of the Mg 1s, Mn 2p, 0.2Cu-0.8Mn specimens had an intact compact corroded
and O 1s peaks. The wide Mg 1s peak was composed surface layer and the lowest biodegradation rate.
of Mg(OH) at 523.4 eV and MgO at 530.4 eV. The two The SLMed ZK30-0.2Cu-xMn is a multiphase
2
constituent peaks of Mn 2p at 654.2 eV and Mn 2p at alloy, in which different microconstituents, that is, the
1/2
3/2
641.9 eV in the Mn 2p indicated the existence of MnO Mg matrix and the second phases may cause strong micro
and MnO , respectively. The O 1s peak was composed galvanic corrosion. As shown in Figure 2, the diffraction
2
of three constituent peaks of MnO at 529.4 eV, MgO at peaks of the Mn phase could be identified only when
x
530.4 eV, and Mg(OH) at 532.4 eV. Consequently, in the Mn content was >0.8 wt.%. This indicates that the
2
addition to Mg(OH) and MgO, manganese oxides were Mn element could completely dissolve in Mg matrix
2
86 International Journal of Bioprinting (2021)–Volume 7, Issue 1