Page 97 - IJB-7-3
P. 97
Mao, et al.
A B
C D
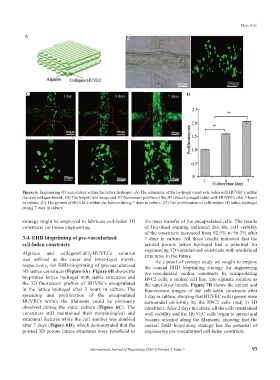
Figure 6. Engineering 3D vasculature within the lattice hydrogel. (A) The schematic of the hydrogel constructs laden with HUVECs within
the core collagen bioink. (B) The bright-field image and 3D fluorescent profiles of the 3D lattice hydrogel laden with HUVECs after 3 hours
in culture. (C) The growth of HUVECs within the lattices during 7 days in culture. (D) The proliferation of cells within 3D lattice hydrogel
during 7 days in culture.
strategy might be employed to fabricate cell-laden 3D the mass transfer of the encapsulated cells. The results
constructs for tissue engineering. of live-dead staining indicated that the cell viability
of the constructs increased from 92.5% to 96.3% after
3.4. EHD bioprinting of pre-vascularized 7 days in culture. All these results indicated that the
cell-laden constructs printed porous lattice hydrogel had a potential for
engineering 3D vascularized constructs with predefined
Alginate and collagen/CaCl /HUVECs solution structures in the future.
2
was utilized as the outer and inner-layer bioink, As a proof of concept study we sought to employ
respectively, for EHD-bioprinting of pre-vascularized the coaxial EHD bioprinting strategy for engineering
3D lattice constructs (Figure 6A). Figure 6B shows the pre-vascularized cardiac constructs by encapsulating
bioprinted lattice hydrogel with stable structures and H9C2 cells, a cardiac cell line, into alginate solution as
the 3D fluorescent profiles of HUVECs encapsulated the outer-layer bioink. Figure 7B shows the optical and
in the lattice hydrogel after 3 hours in culture. The fluorescence images of the cell-laden constructs after
spreading and proliferation of the encapsulated 1 day in culture, showing that HUVEC cells (green) were
HUVECs within the filaments could be obviously surrounded uniformly by the H9C2 cells (red) in 3D
observed during the static culture (Figure 6C). The constructs. After 2 days in culture, all the cells maintained
constructs still maintained their morphological and well viability and the HUVEC cells began to spread and
structural features while the cell number was doubled became oriented along the filaments, showing that the
after 7 days (Figure 6D), which demonstrated that the coaxial EHD bioprinting strategy has the potential of
printed 3D porous lattice structures were beneficial to engineering pre-vascularized cell-laden constructs.
International Journal of Bioprinting (2021)–Volume 7, Issue 3 93