Page 110 - IJB-7-4
P. 110
CTP Scaffolds Treated Bone Defects
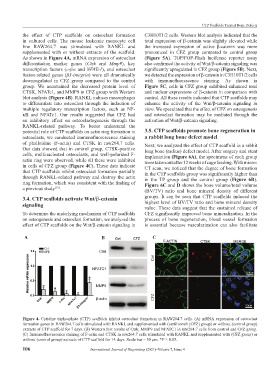
the effect of CTP scaffolds on osteoclast formation C3H10T1/2 cells. Western blot analysis indicated that the
in cultured cells. The mouse leukemic monocyte cell total expression of β-catenin was slightly elevated while
line RAW264.7 was stimulated with RANKL and the increased expression of active β-catenin was more
supplemented with or without extracts of the scaffold. pronounced in CFZ group compared to control group
As shown in Figure 4A, mRNA expression of osteoclast (Figure 5A). TOP/FOP-Flash luciferase reporter assay
differentiation marker genes (Ctsk and Mmp9), key also confirmed the activity of Wnt/β-catenin signaling was
transcription factors (c-fos and NFATc1), and osteoclast significantly upregulated in CFZ group (Figure 5B). Next,
fusion-related genes (β3-integrin) were all dramatically we detected the expression of β-catenin in C3H10T1/2 cells
downregulated in CFZ group compared to the control with immunofluorescence staining. As shown in
group. We ascertained the decreased protein level of Figure 5C, cells in CFZ group exhibited enhanced total
CTSK, NFATc1, and MMP9 in CFZ group with Western and nuclear expressions of β-catenin in comparison with
blot analysis (Figure 4B). RANKL induces macrophages control. All these results indicated that CTP scaffolds may
to differentiate into osteoclast through the induction of enhance the activity of the Wnt/β‐catenin signaling in
multiple regulatory transcription factors, such as NF‐ vitro. We speculated that the effect of CFZ on osteogenesis
κB and NFATc1. Our results suggested that CFZ had and osteoclast formation may be mediated through the
an inhibitory effect on osteoclastogenesis through the activation of Wnt/β-catenin signaling.
RANKL-related pathway. To better understand the
potential role of CTP scaffolds on actin ring formation in 3.5. CTP scaffolds promote bone regeneration in
osteoclasts, we conducted immunofluorescence staining a rabbit long bone defect model
of phalloidine (F-actin) and CTSK in raw264.7 cells. Next, we analyzed the effect of CTP scaffold in a rabbit
Our data showed that in control group, CTSK-positive long bone (radius) defect model. After surgery and stent
cells, multinucleated osteoclasts, and well‐polarized F‐ implantation (Figure 6A), the specimens of each group
actin ring were observed, while all these were inhibited were taken out after 12 weeks of cage feeding. With micro-
in cells of CFZ group (Figure 4C). These data indicate CT scan, we noticed that the degree of bone formation
that CTP scaffolds inhibit osteoclast formation partially in the CTP scaffolds group was significantly higher than
through RANKL-related pathway and destroy the actin in the TP group and the control group (Figure 6B).
ring formation, which was consistent with the finding of Figure 6C and D shows the bone volume/total volume
a previous study . (BV/TV) ratio and bone mineral density of different
[15]
3.4. CTP scaffolds activate Wnt/β-catenin groups. It can be seen that CTP scaffolds induced the
signaling highest level of BV/TV ratio and bone mineral density
value. These data suggest that the sustained release of
To determine the underlying mechanism of CTP scaffolds CFZ significantly improved bone mineralization. In the
on osteogenesis and osteoclast formation, we analyzed the process of bone regeneration, blood vessel formation
effect of CTP scaffolds on the Wnt/β-catenin signaling in is essential because vascularization can also facilitate
A B C
Figure 4. Cytidine triphosphate (CTP) scaffolds inhibit osteoclast formation in RAW264.7 cells. (A) mRNA expression of osteoclast
formation genes in RAW264.7 cells stimulated with RANKL and supplemented with (carfilzomib [CFZ] group) or without (control group)
extracts of CTP scaffold for 7 days. (B) Western blot results of Ctsk, MMP9 and NFATC1 in raw264.7 cells from control and CFZ group.
(C) Immunofluorescence staining of F-actin and CTSK in raw264.7 cells stimulated with RANKL and supplemented with (CFZ group) or
without (control group) extracts of CTP scaffold for 14 days. Scale bar = 50 μm. *P < 0.05.
106 International Journal of Bioprinting (2021)–Volume 7, Issue 4