Page 56 - IJB-7-4
P. 56
Filament Structure, 3D printing, Bone Repair Scaffolds
A D
B
C E
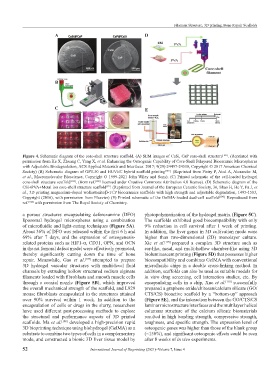
Figure 4. Schematic diagram of the core-shell structure scaffold. (A) SEM images of CaSi, CaP core-shell structure [101] . (Reprinted with
permission from Ke X, Zhuang C, Yang X, et al. Enhancing the Osteogenic Capability of Core-Shell Bilayered Bioceramic Microspheres
with Adjustable Biodegradation, ACS Applied Materials and Interfaces. 2017; 9(29):24497-24510, Copyright © 2017 American Chemical
Society) (B) Schematic diagram of GPT-50 and HUVEC hybrid scaffold printing [102] . (Reprinted from Pistry P, Aied A, Alexander M,
et al., Macromolecular Bioscience, Copyright © 1999-2021 John Wiley and Sons). (C) Printed schematic of the cell-loaded hydrogel
core-shell structure scaffold [103] . (from ref. [103] licensed under Creative Commons Attribution 4.0 license). (D) Schematic diagram of the
CSi+PVA+Metal ion core-shell structure scaffold . (Reprinted from Journal of the European Ceramic Society, 36, Shao H, He Y, Fu J, et
[92]
al., 3D printing magnesium-doped wollastonite/β-TCP bioceramics scaffolds with high strength and adjustable degradation, 1495-1503,
Copyright (2016), with permission from Elsevier) (E) Printed schematic of the GelMA-loaded dual-cell scaffold [104] . Reproduced from
ref. [104] with permission from The Royal Society of Chemistry.
a porous structures encapsulating deferoxamine (DFO) photopolymerization of the hydrogel matrix (Figure 5C).
liposomal hydrogel microspheres using a combination The scaffolds exhibited good biocompatibility with only
of microfluidic and light-curing techniques (Figure 5A). 9% reduction in cell survival after 1 week of printing.
About 36% of DFO was released within the first 6 h and In addition, the liver genes in 3D cultivation mode were
69% after 7 days, and the expression of osteogenesis- higher than two-dimensional (2D) monolayer culture.
related proteins such as HIF1-α, CD31, OPN, and OCN Xie et al. [109] prepared a complex 3D structure such as
in the rat femoral defect model were effectively promoted, ear-like, nasal, and multi-hollow chamber-like using 3D
thereby significantly cutting down the time of bone bioluminescent printing (Figure 5D) that possesses higher
repair. Meanwhile, Gao et al. [107] attempted to prepare biocompatibility and combines GelMA with conventional
3D hydrogel vascular structures with multi-level fluid microfluidic chips in a double cross-linking method. In
channels by extruding hollow structured sodium alginate addition, scaffolds can also be used as suitable models for
filaments loaded with fibroblasts and smooth muscle cells in vitro drug screening, cell interaction studies, etc. By
through a coaxial nozzle (Figure 5B), which improved encapsulating cells in a chip, Xue et al. [110] successfully
the overall mechanical strength of the scaffold, and L929 prepared a graphene oxide/chitosan/calcium silicate (GO/
mouse fibroblasts encapsulated in the structures attained CTS/CS) bioactive scaffold by a “bottom-up” approach
over 90% survival within 1 week. In addition to the (Figure 5E), and the interaction between the GO/CTS/CS
encapsulation of cells or drugs in the slurry, researchers laminar microstructure interfaces and the multilayer helical
have used different post-processing methods to explore columnar structure of the calcium silicate biomaterials
the structural and performance aspects of 3D printed resulted in high bending strength, compressive strength,
scaffolds. Ma et al. [108] developed a high-precision rapid toughness, and specific strength. The expression level of
3D bioprinting technique using biohydrogel (GelMA) as a osteogenic genes was higher than those of the blank group
substrate to combine two types of cells in a complementary (~150%), and significant osteogenic effects could be seen
mode, and constructed a bionic 3D liver tissue model by after 8 weeks of in vivo experiments.
52 International Journal of Bioprinting (2021)–Volume 7, Issue 4