Page 45 - IJB-8-2
P. 45
Jiao, et al.
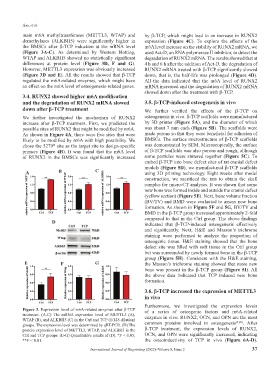
main m6A methyltransferases (METTL3, WTAP) and by β-TCP, which might lead to an increase in RUNX2
demethylases (ALKBH5) were significantly higher in expression (Figure 4C). To explore the effects of the
the BMSCs after β-TCP induction at the mRNA level m6A level increase on the stability of RUNX2 mRNA, we
(Figure 3A-C). As determined by Western blotting, used Act-D, an RNA polymerase II inhibitor, to detect the
WTAP and ALKBH5 showed no statistically significant degradation of RUNX2 mRNA. The results showed that at
differences at protein level (Figure 3D, F and G). 4 h and 6 h after the addition of Act-D, the degradation of
However, METTL3 expression was obviously increased RUNX2 mRNA treated with β-TCP significantly slowed
(Figure 3D and E). All the results showed that β-TCP down, that is, the half-life was prolonged (Figure 4D).
regulated the m6A-related enzymes, which might have All the data indicated that the m6A level of RUNX2
an effect on the m6A level of osteogenesis-related genes. mRNA increased and the degradation of RUNX2 mRNA
slowed down after the treatment with β-TCP.
3.4. RUNX2 showed higher m6A modification
and the degradation of RUNX2 mRNA slowed 3.5. β-TCP-induced osteogenesis in vivo
down after β-TCP treatment We further verified the effects of the β-TCP on
We further investigated the mechanism of RUNX2 osteogenesis in vivo. β-TCP scaffolds were manufactured
increase after β-TCP treatment. First, we predicted the by 3D printer (Figure 5A), and the diameter of which
possible sites of RUNX2 that might be modified by m6A. was about 7 mm each (Figure 5B). The scaffolds were
As shown in Figure 4A, there were five sites that were made porous so that they were beneficial for adhesion of
likely to be modified by m6A with high possibility. We BMSCs. The surface microstructure of β-TCP scaffolds
chose the 5279 site as the target site to design-specific was demonstrated by SEM. Microscopically, the surface
th
primers (Figure 4B). It was found that the m6A level of β-TCP scaffolds was also porous and rough, although
of RUNX2 in the BMSCs was significantly increased some particles were sintered together (Figure 5C). To
embed β-TCP into bone defect sites of rat cranial defect
models (Figure 5D), we manufactured β-TCP scaffolds
A B C using 3D printing technology. Eight weeks after model
construction, we sacrificed the rats to obtain the skull
samples for micro-CT analyses. It was shown that some
new bone was formed inside and outside the cranial defect
(yellow section) (Figure 5E). Next, bone volume fraction
(BV/TV) and BMD were evaluated to assess new bone
formation. As shown in Figure 5F and 5G, BV/TV and
BMD in the β-TCP group increased approximately 2-fold
compared to that in the Ctrl group. The above findings
D indicated that β-TCP-induced osteogenesis effectively
and significantly. Next, H&E and Masson’s trichrome
staining were performed to analyze the proportion of
osteogenic tissue. H&E staining showed that the bone
defect site was filled with soft tissue in the Ctrl group
but was surrounded by newly formed bone in the β-TCP
group (Figure 5H). Consistent with the H&E staining,
E F G the Masson’s trichrome staining showed that more new
bone was present in the β-TCP group (Figure 5I). All
the above data indicated that TCP induced new bone
formation.
3.6. β-TCP increased the expression of METTL3
in vivo
Furthermore, we investigated the expression levels
Figure 3. Expression level of m6A-related enzymes after β-TCP of a series of osteogenic factors and m6A-related
treatment. (A-C) The mRNA expression level of METTL3 (A),
WTAP (B), and ALKBH5 (C) in the Ctrl and TCP (1/128 dilution) enzymes in vivo. RUNX2, OCN, and OPN are the most
[3,26]
groups. The expression level was determined by qRT-PCR. (D) The common proteins involved in osteogenesis . After
protein expression level of METTL3, WTAP, and ALKBH5 in the β-TCP treatment, the expression levels of RUNX2,
Ctrl and TCP groups. (E-G) Quantitative results of (D). *P < 0.05; OCN, and OPN were significantly increased, indicating
**P < 0.01. the osteoinductivity of TCP in vivo (Figure 6A-D).
International Journal of Bioprinting (2022)–Volume 8, Issue 2 37