Page 97 - IJB-8-2
P. 97
Wang, et al.
A B
C D
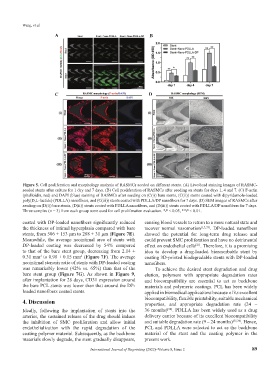
Figure 5. Cell proliferation and morphology analysis of RASMCs seeded on different stents. (A) Live/dead staining images of RASMC-
seeded stents after culture for 1 day and 7 days. (B) Cell proliferation of RASMCs after seeding on stents for days 1, 4 and 7. (C) F-actin
(phalloidin, red) and DAPI (blue) staining of RASMCs after seeding on (C(i)) bare stents, (C(ii)) stents coated with dipyridamole-loaded
poly(D,L-lactide) (PDLLA) nanofibers, and (C(iii)) stents coated with PDLLA/DP nanofibers for 7 days. (D) SEM images of RASMCs after
seeding on (D(i)) bare stents, (D(ii)) stents coated with PDLLA nanofibers, and (D(iii)) stents coated with PDLLA/DP nanofibers for 7 days.
Three samples (n = 3) from each group were used for cell proliferation evaluation. *P < 0.05, **P < 0.01.
coated with DP-loaded nanofibers significantly reduced causing blood vessels to return to a more natural state and
the thickness of intimal hyperplasia compared with bare recover normal vasomotion [1,3,33] . DP-loaded nanofibers
stents, from 506 ± 153 μm to 288 ± 31 μm (Figure 7E). showed the potential for long-term drug release and
Meanwhile, the average neointimal area of stents with could prevent SMC proliferation and have no detrimental
DP-loaded coating was decreased by 54% compared effect on endothelial cells . Therefore, it is a promising
[21]
to that of the bare stent group, decreasing from 2.14 ± idea to develop a drug-loaded bioresorbable stent by
0.31 mm to 0.98 ± 0.15 mm (Figure 7F). The average coating 3D-printed biodegradable stents with DP-loaded
2
2
neointimal stenosis ratio of stents with DP-loaded coating nanofibers.
was remarkably lower (42% vs. 65%) than that of the To achieve the desired stent degradation and drug
bare stent group (Figure 7G). As shown in Figure 9, elution, polymers with appropriate degradation rates
after implantation for 28 days, CD31 expression around and biocompatibility are essential to act as backbone
the bare PCL stents was lower than that around the DP- materials and polymeric coatings. PCL has been widely
loaded nanofibers coated stents. applied in biomedical applications because of its excellent
4. Discussion biocompatibility, flexible printability, suitable mechanical
properties, and appropriate degradation rate (24 –
[34]
Ideally, following the implantation of stents into the 36 months) . PDLLA has been widely used as a drug
arteries, the sustained release of the drug should induce delivery carrier because of its excellent biocompatibility
the inhibition of SMC proliferation and allow initial and suitable degradation rate (9 – 24 months) [35,36] . Hence,
endothelialization with the rapid degradation of the PCL and PDLLA were selected to act as the backbone
coating polymer material. Subsequently, as the backbone material of the stent and the coating polymer in the
materials slowly degrade, the stent gradually disappears, present work.
International Journal of Bioprinting (2022)–Volume 8, Issue 2 89