Page 201 - IJB-9-1
P. 201
International Journal of Bioprinting 3D bioprinting of tissue with carbon nanomaterials
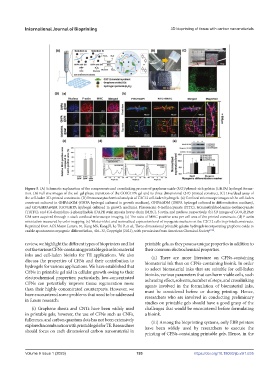
Figure 5. (A) Schematic explanation of the components and crosslinking process of graphene oxide (GO)/phenol-rich gelatin (GHPA) hydrogel forma-
tion. (B) Full size images of the sol–gel phase transition of the GO/GHPA gel and its three-dimensional (3D)-printed construct. (C) Live/dead assay of
the cell-laden 3D-printed constructs. (D) Immunocytochemical analysis of C2C12 cell-laden hydrogels. (a) Confocal microscope images of the cell-laden
construct cultured in GHPA@GM (GHPA hydrogel cultured in growth medium), GHPA@DM (GHPA hydrogel cultured in differentiation medium),
and GO/GHPA@GM (GO/GHPA hydrogel cultured in growth medium). Fluorescein-5-isothiocyanate (FITC), tetramethylrhodamine-isothiocyanate
(TRITC), and 4′,6-diamidino-2-phenylindole (DAPI) stain myosin heavy chain (MHC), f-actin, and nucleus, respectively. (b) 3D images of GO/GHPA@
GM were acquired through z-stack confocal microscope imaging. (c) The ratio of MHC-positive area per cell area of the printed constructs. (d) F-actin
orientation measured by color mapping. (e) Western blot and normalized expression level of myogenic markers on the C2C12 cells in printed constructs.
Reprinted from ACS Macro Letters, 10, Kang MS, Kang JI, Le Thi P, et al., Three-dimensional printable gelatin hydrogels incorporating graphene oxide to
enable spontaneous myogenic differentiation, 426–32, Copyright (2021), with permission from American Chemical Society [106] .
review, we highlight the different types of bioprinters and list printable gels, as they possess unique properties in addition to
out the various CFNs-containing printable gels as biomaterial their common electrochemical properties.
inks and cell-laden bioinks for TE applications. We also (ii) There are more literature on CFNs-containing
discuss the properties of CFNs and their contribution to biomaterial ink than on CFNs-containing bioink. In order
hydrogels for various applications. We have established that to select biomaterial inks that are suitable for cell-laden
CFNs in printable gel aid in cellular growth owing to their bioinks, various parameters that can harm viable cells, such
electrochemical properties; particularly, low-concentrated as heating effect, solvents, number of steps, and crosslinking
CFNs can potentially improve tissue regeneration more agents involved in the formulation of biomaterial inks,
than their highly concentrated counterparts. However, we must be considered before or during printing. Hence,
have encountered some problems that need to be addressed researchers who are involved in conducting preliminary
in future research:
studies on printable gels should have a good grasp of the
(i) Graphene sheets and CNTs have been widely used challenges that would be encountered before formulating
in printable gels; however, the use of CFNs such as CNFs, a bioink.
fullerenes, and carbon quantum dots has not been extensively (iii) Among the bioprinting systems, only EBB printers
explored in combination with printable gels for TE. Researchers have been widely used by researchers to execute the
should focus on each dimensional carbon nanomaterial in printing of CFNs-containing printable gels. Hence, in the
Volume 9 Issue 1 (2023) 193 https://doi.org/10.18063/ijb.v9i1.635