Page 370 - IJB-9-1
P. 370
International Journal of Bioprinting Micro/nano-3D hemostats for rapid wound healing
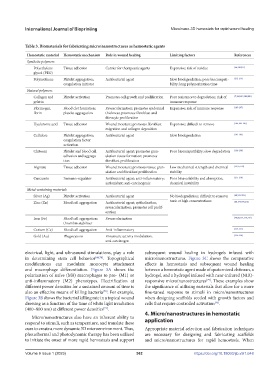
Table 3. Biomaterials for fabricating micro/nanostructures as hemostatic agents
Hemostatic material Hemostatic mechanism Role in wound healing Limiting factors References
Synthetic polymers
Polyethylene Tissue adhesion Carrier for therapeutic agents Expensive; risk of residue [64,210,211]
glycol (PEG)
Polyurethane Platelet aggregation; Antibacterial agent Slow biodegradation; poor biocompati- [212-214]
coagulation initiator bility; long polymerization time
Natural polymers
Collagen and Platelet activation Promotes cell growth and proliferation Poor resistance to degradation; risk of [7,124,183,184,200]
gelatin immune response
Fibrinogen, Blood clot formation; Revascularization; promotes epidermal Expensive; risk of immune response [185-187]
fibrin platelet aggregation thickness; promotes fibroblast and
fibrocyte proliferation
Hyaluronic acid Tissue adhesion Wound moisture; promotes fibroblast Expensive; difficult to remove [186,188-190]
migration and collagen deposition
Cellulose Platelet aggregation; Antibacterial agent Slow biodegradation [191-193]
coagulation factor
activation
Chitosan Platelet and blood cell Antibacterial agent; promotes gran- Poor biocompatibility; slow degradation [194-198]
adhesion and aggrega- ulation tissue formation; promotes
tion fibroblast proliferation
Alginate Tissue adhesion Wound moisture; promotes tissue gran- Low mechanical strength and chemical [53,55,199]
ulation and fibroblast proliferation stability
Curcumin Immuno-regulator Antibacterial agent; anti-inflammatory; Poor bioavailability and absorption; [215-218]
antioxidant; anti-carcinogenic chemical instability
Metal-containing materials
Silver (Ag) Platelet activation Antibacterial agent No biodegradation; difficult to remove; [68,219-222]
Zinc (Zn) Blood cell aggregation Antibacterial agent; epithelization; toxic at high concentrations [68,174,190,216]
revascularization; promotes cell prolif-
eration
Iron (Fe) Blood cell aggregation; Revascularization [40,68,171,215,219]
thrombin stabilizer
Cerium (Ce) Blood cell aggregation Anti-inflammatory [223-225]
Gold (Au) Phagocytosis Enzymatic activity modulation, [226-228]
anti-carcinogen
electrical, light, and ultrasound stimulations, play a role subsequent wound healing in hydrogels infused with
in determining stem cell behavior [44,74] . Topographical micro/nanostructures. Figure 3C shows the comparative
modifications can modulate monocyte attachment effects in hemostasis and subsequent wound healing
and macrophage differentiation. Figure 3A shows the between a hemostatic agent made of quaternized chitosan, a
polarization of naïve (M0) macrophages to pro- (M1) or hydrogel, and a hydrogel infused with near-infrared (NIR)-
anti-inflammatory (M2) phenotypes. Electrification at responsive micro/nanostructures . These examples show
[79]
different power densities for a sustained amount of time is the significance of utilizing materials that allow for a more
also an effective means of killing bacteria . For example, fine-tuned response to stimuli in micro/nanostructures
[70]
Figure 3B shows the bacterial killing rate in a topical wound when designing scaffolds seeded with growth factors and
dressing as a function of the time of white light irradiation cells that require controlled activation .
[45]
(400–800 nm) at different power densities .
[71]
4. Micro/nanostructures in hemostatic
Micro/nanostructures also have an inherent ability to
respond to stimuli, such as temperature, and translate these application
cues to create a more dynamic 3D microenvironment. Thus, Appropriate material selection and fabrication techniques
photothermal and photodynamic therapy has been utilized are necessary for designing and fabricating scaffolds
to initiate the onset of more rapid hemostasis and support and micro/nanostructures for rapid hemostasis. When
V 362 https://doi.org/10.18063/ijb.v9i1.648
Volume 9 Issue 1 (2023)olume 9 Issue 1 (2023)