Page 81 - IJB-9-1
P. 81
International Journal of Bioprinting Customized 3D nasal masks for ELBW infants
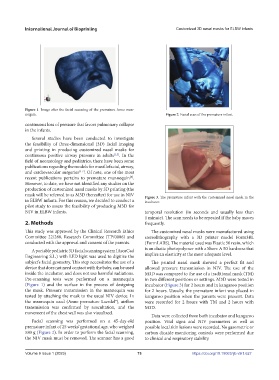
Figure 1. Image after the facial scanning of the premature Anne man-
nequin. Figure 2. Facial scan of the premature infant.
continuous loss of pressure that favors pulmonary collapse
in the infants.
Several studies have been conducted to investigate
the feasibility of three-dimensional (3D) facial imaging
and printing in producing customized nasal masks for
continuous positive airway pressure in adults [1,2] . In the
field of neonatology and pediatrics, there have been some
publications regarding the models for maxillofacial, airway,
and cardiovascular surgeries [3-7] . Of note, one of the most
recent publications pertains to premature mannequin .
[8]
However, to date, we have not identified any studies on the
production of customized nasal masks by 3D printing (the
mask will be referred to as M3D thereafter) for use in NIV Figure 3. The premature infant with the customized nasal mask in the
in ELBW infants. For this reason, we decided to conduct a incubator.
pilot study to assess the feasibility of producing M3D for
NIV in ELBW infants. temporal resolution (in seconds and usually less than
1 minute). The scan needs to be repeated if the baby moves
2. Methods frequently.
This study was approved by the Clinical Research Ethics The customized nasal masks were manufactured using
Committee 22/288, Research Committee (TP/0086) and stereolithography with a 3D printer model Form3BL
conducted with the approval and consent of the parents. (FormLABS). The material used was Elastic 50 resin, which
A portable pediatric 3D facial scanning system (AsorCad is an elastic photopolymer with a Shore A 50 hardness that
Engineering S.L.) with LED light was used to digitize the implies an elasticity at the most adequate level.
subject’s facial geometry. This step necessitates the use of a The printed nasal mask showed a perfect fit and
device that does not need contact with the baby, can be used allowed pressure transmission in NIV. The use of the
inside the incubator, and does not use harmful radiations. M3D was compared to the use of a traditional mask (TM)
Pre-scanning tests were performed on a mannequin in two different positions or settings. M3D were tested in
(Figure 1) and the surface in the process of designing incubator (Figure 3) for 2 hours and in kangaroo position
the mask. Pressure transmission in the mannequin was for 2 hours. Usually, the premature infant was placed in
tested by attaching the mask to the usual NIV device. In kangaroo position when the parents were present. Data
the mannequin used (Anne premature Laerdal®), airflow were recorded for 2 hours with TM and 2 hours with
transmission was confirmed by auscultation, and the M3D.
movement of the chest wall was also visualized.
Data were collected from both incubator and kangaroo
Facial scanning was performed on a 45-day-old position. Vital signs and NIV parameters as well as
premature infant of 25 weeks’ gestational age, who weighed possible local skin lesions were recorded. No gasometric or
580 g (Figure 2). In order to perform the facial scanning, carbon dioxide monitoring controls were performed due
the NIV mask must be removed. The scanner has a good to clinical and respiratory stability.
Volume 9 Issue 1 (2023)olume 9 Issue 1 (2023)
V 73 https://doi.org/10.18063/ijb.v9i1.627