Page 86 - IJB-9-2
P. 86
International Journal of Bioprinting Steam-sterilized and degradable FFF-printed PLA/PHA surgical guides
Figure 1. Workflow of this study to compare sterilized and non-sterilized 3D-printed materials for implant placement guides.
2. Materials and methods A B
2.1. Model creation, model printing, and virtual
surgical planning
An upper jaw plaster model was scanned with a
laboratory scanner (inEos X5, Dentsply Sirona Inc.,
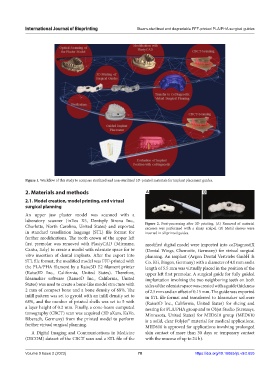
Charlotte, North Carolina, United States) and exported Figure 2. Post-processing after 3D printing. (A) Removal of material
excesses was performed with a sharp scalpel. (B) Metal sleeves were
in standard tessellation language (STL) file format for inserted in all printed guides.
further modifications. The tooth crown of the upper left
first premolar was removed with PlastyCAD (3diemme, modified digital model were imported into coDiagnostiX
Cantu, Italy) to create a model with edentate space for in (Dental Wings, Chemnitz, Germany) for virtual surgical
vitro insertion of dental implants. After the export into planning. An implant (Argon Dental Vertriebs GmbH &
STL file format, the modified model was FFF-printed with Co. KG, Bingen, Germany) with a diameter of 4.0 mm and a
the PLA/PHA filament by a Raise3D E2 filament printer length of 5.5 mm was virtually placed in the position of the
(Raise3D Inc., California, United States). Therefore, upper left first premolar. A surgical guide for fully guided
Ideamaker software (Raise3D Inc., California, United implantation involving the two neighboring teeth on both
States) was used to create a bone-like model structure with sides of the edentate space was created with a guide thickness
2 mm of compact bone and a bone density of 68%. The of 2.5 mm and an offset of 0.15 mm. The guide was exported
infill pattern was set to gyroid with an infill density set to in STL file format and transferred to Ideamaker software
68%, and the number of printed shells was set to 5 with (Raise3D Inc., California, United States) for slicing and
a layer height of 0.2 mm. Finally, a cone-beam computed nesting for PLA/PHA group and to Objet Studio (Stratasys,
tomography (CBCT) scan was acquired (3D aXam, KaVo, Minnesota, United States) for MED610 group (MED610
Biberach, Germany) from the printed model to perform is a solid, clear PolyJet™ material for medical applications.
further virtual surgical planning. MED610 is approved for applications involving prolonged
A Digital Imaging and Communications in Medicine skin contact of more than 30 days or temporary contact
(DICOM) dataset of the CBCT scan and a STL file of the with the mucosa of up to 24 h).
Volume 9 Issue 2 (2023) 78 https://doi.org/10.18063/ijb.v9i2.655