Page 333 - IJB-9-3
P. 333
International Journal of Bioprinting New fibrillar collagen for 3D printing and bioprinting
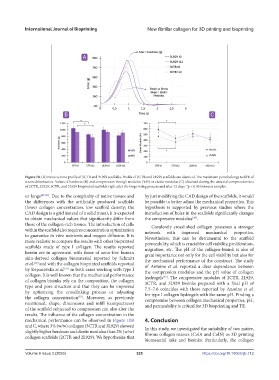
Figure 10. (A) Force vs time profile of 3CTR and 3L929 scaffolds. Profile of 2CTR and 2L929 scaffolds are identical. The maximum point belongs to 80% of
strain deformation. Values of hardness (B) and compression Young’s modulus (YM) or elastic modulus (C) obtained during the uniaxial compression test
of 2CTR, 2L929, 3CTR, and 3L929 bioprinted scaffolds right after the bioprinting process and after 12 days. *p < 0.05 between samples.
or lungs [47-49] . Due to the complexity of native tissues and by just modifying the CAD design of the scaffolds, it would
the differences with the artificially produced scaffolds be possible to better adjust the mechanical properties. This
(lower collagen concentration, low scaffold density; the hypothesis is supported by previous studies where the
CAD design is a grid instead of a solid mass), it is expected introduction of holes in the scaffolds significantly changes
to obtain mechanical values that significantly differ from the compressive modulus .
[48]
those of the collagen-rich tissues. The introduction of cells Covalently crosslinked collagen possesses a stronger
within the scaffold also requires concentration optimization network with improved mechanical properties.
to guarantee in vitro nutrients and oxygen diffusion. It is Nevertheless, this can be detrimental to the scaffold
more realistic to compare the results with other bioprinted permeability, which is crucial for cell viability, proliferation,
scaffolds made of type I collagen. The results reported migration, etc. The pH of the collagen-bioink is also of
herein are in agreement with those of xeno-free human great importance not only for the cell viability but also for
skin-derived collagen biomaterial reported by Schmitt the mechanical performance of the construct. The study
et al. and with the collagen bioprinted scaffolds reported of Antoine et al. reported a clear dependence between
[50]
by Stepanovska et al. in both cases working with type I the compression modulus and the pH value of collagen
[51]
collagen. It is well known that the mechanical performance hydrogels . The compressive modulus of 2CTR, 2L929,
[53]
of collagen bioinks rely on the composition, the collagen 3CTR, and 3L929 bioinks prepared with a final pH of
type and pore structure and that they can be improved 7.5–7.6 coincides with those reported by Antoine et al.
by optimizing the crosslinking process or adjusting for type I collagen hydrogels with the same pH. Finding a
the collagen concentration . Moreover, as previously compromise between collagen mechanical properties, pH,
[52]
mentioned, shape, dimensions and infill (compactness) and permeability is critical for 3D bioprinting and TE.
of the scaffold subjected to compression can also alter the
results. The influence of the collagen concentration in the
mechanical performance can be observed in Figure 10B 4. Conclusion
and C, where 3% (w/w) collagen (3CTR and 3L929) showed In this study, we investigated the suitability of two native,
slightly higher hardness and elastic modulus than 2% (w/w) fibrous collagen masses (ColA and ColN) as 3D printing
collagen scaffolds (2CTR and 2L929). We hypothesize that
biomaterial inks and bioinks. Particularly, the collagen
Volume 9 Issue 3 (2023) 325 https://doi.org/10.18063/ijb.712