Page 92 - IJB-9-3
P. 92
International Journal of Bioprinting Biocompatible 3D printing photosensitive resin
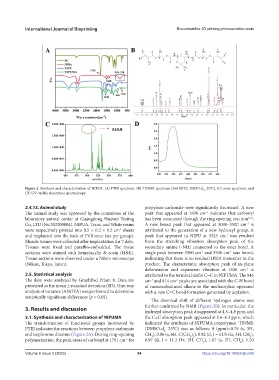
Figure 2. Synthesis and characterization of NIPUA. (A) FTIR spectrum, (B) HNMR spectrum (400 MHZ, DMSO-d , 25°C), (C) mass spectrum, and
1
6
(D) UV–visible absorption spectroscopy.
2.4.13. Animal study propylene carbonate were significantly decreased. A new
The animal study was approved by the committee of the peak that appeared at 1698 cm indicates that carbonyl
-1
laboratory animal center at Guangdong Huawei Testing has been consumed through the ring opening reaction .
[21]
Co, LTD (No.202209004). NIPUA, Trans, and White resins A new broad peak that appeared at 3000–3500 cm is
-1
were respectively printed into 0.5 × 0.2 × 0.2 cm sheets attributed to the generation of a new hydroxyl group. A
3
and implanted into the back of FVB mice (six per group). peak that appeared in NIPU at 3323 cm was resulted
-1
Muscle tissues were collected after implantation for 7 days. from the stretching vibration absorption peak of the
Tissues were fixed and paraffin-embedded. The tissue secondary amine (-NH) connected to the ester bond. A
sections were stained with hematoxylin & eosin (H&E). single peak between 3300 cm and 3500 cm was found,
-1
-1
Tissue sections were observed under a Nikon microscope indicating that there is no residual IPDA monomer in the
(Nikon, Tokyo, Japan). product. The characteristic absorption peak of in-plane
deformation and expansion vibration at 1636 cm is
-1
2.5. Statistical analysis attributed to the terminal olefin C=C in NIPUMA. The 946
The data were analyzed by GraphPad Prism 8. Data are cm and 814 cm peaks are associated with the C-H bond
-1
-1
presented as the mean ± standard deviation (SD). One-way of monosubstituted alkene in the methacrylate appeared
analysis of variance (ANOVA) was performed to determine with a new C=C bond formation generated by acylation.
statistically significant differences (p < 0.05).
The chemical shift of different hydrogen atoms was
3. Results and discussion further confirmed by NMR (Figure 2B). In particular, the
hydroxyl absorption peak disappeared at 4.5–4.8 ppm and
3.1. Synthesis and characterization of NIPUMA the C=C absorption peak appeared at 5.6–6.3 ppm, which
The transformation of functional groups monitored by indicated the synthesis of NIPUMA-prepolymer. HNMR
1
FTIR indicates the reactions between propylene carbonate (DMSO-d , 25°C) was as follows: δ (ppm)=0.79 (s, 3H,
6
and isophorone diamine (Figure 2A). During ring-opening CH ), 0.86 (s, 6H, C(CH ) ), 0.92 (d, J = 11.8 Hz, 3H, CH ),
3 2
3
3
polymerization, the peak areas of carbonyl at 1791 cm for 0.97 (d, J = 11.2 Hz, 3H, CH ), 1.07 (s, 2H, CH ), 1.20
-1
2
3
Volume 9 Issue 3 (2023) 84 https://doi.org/10.18063/ijb.684