Page 44 - IJB-9-4
P. 44
International Journal of Bioprinting 3D-Bioprinted human lipoaspirate-derived cell-laden skin constructs
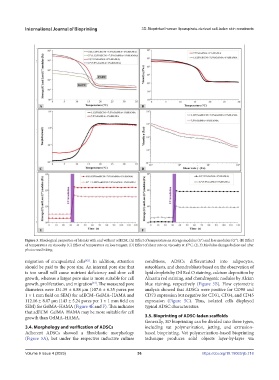
Figure 3. Rheological properties of bioinks with and without adECM. (A) Effect of temperature on storage modulus (G′) and loss modulus (G″). (B) Effect
of temperature on viscosity. (C) Effect of temperature on loss tangent. (D) Effect of shear rate on viscosity at 17°C. (E, F) Modulus changes before and after
photocrosslinking.
[42]
migration of encapsulated cells . In addition, attention conditions, ADSCs differentiated into adipocytes,
should be paid to the pore size. An internal pore size that osteoblasts, and chondroblasts based on the observation of
is too small will cause nutrient deficiency and slow cell lipid droplets by Oil Red O staining, calcium deposition by
growth, whereas a larger pore size is more suitable for cell Alizarin red staining, and chondrogenic nodules by Alcian
[43]
growth, proliferation, and migration . The measured pore blue staining, respectively (Figure 5B). Flow cytometric
diameters were 131.39 ± 6.88 µm (107.6 ± 6.35 pores per analysis showed that ADSCs were positive for CD90 and
1 × 1 mm field on SEM) for adECM–GelMA–HAMA and CD73 expression but negative for CD31, CD34, and CD45
112.16 ± 8.07 µm (143 ± 5.24 pores per 1 × 1 mm field on expression (Figure 5C). Thus, isolated cells displayed
SEM) for GelMA–HAMA (Figure 4E and F). This indicates typical ADSC characteristics.
that adECM–GelMA–HAMA may be more suitable for cell
growth than GelMA–HAMA. 3.5. Bioprinting of ADSC-laden scaffolds
Generally, 3D bioprinting can be divided into three types,
3.4. Morphology and verification of ADSCs including vat polymerization, jetting, and extrusion-
Adherent ADSCs showed a fibroblastic morphology based bioprinting. Vat polymerization-based bioprinting
(Figure 5A), but under the respective inductive culture technique produces solid objects layer-by-layer via
Volume 9 Issue 4 (2023) 36 https://doi.org/10.18063/ijb.718