Page 45 - IJB-9-4
P. 45
International Journal of Bioprinting 3D-Bioprinted human lipoaspirate-derived cell-laden skin constructs
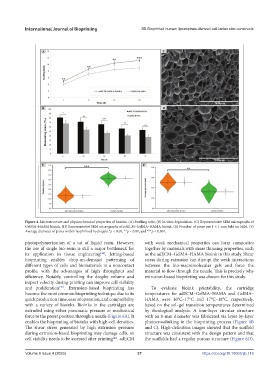
Figure 4. Microstructure and physicochemical properties of bioinks. (A) Swelling ratio. (B) In vitro degradation. (C) Representative SEM micrographs of
GelMA–HAMA bioink. (D) Representative SEM micrographs of adECM–GelMA–HAMA bioink. (E) Number of pores per 1 × 1 mm field on SEM. (F)
Average diameter of pores within lyophilized hydrogels.*p < 0.05, **p < 0.01, and ***p < 0.001.
photopolymerization of a vat of liquid resin. However, with weak mechanical properties can form composites
the use of single bio-resin is still a major bottleneck for together by materials with shear thinning properties, such
its application in tissue engineering . Jetting-based as the adECM–GelMA–HAMA bioink in this study. Shear
[44]
bioprinting enables drop-on-demand patterning of stress during extrusion can disrupt the weak interactions
different types of cells and biomaterials in a noncontact between the bio-macromolecular gels and force the
profile, with the advantages of high throughput and material to flow through the nozzle. This is precisely why
efficiency. Notably, controlling the droplet volume and extrusion-based bioprinting was chosen for this study.
impact velocity during printing can improve cell viability
and proliferation . Extrusion-based bioprinting has To evaluate bioink printability, the cartridge
[45]
become the most common bioprinting technique due to its temperatures for adECM–GelMA–HAMA and GelMA–
quick production time, ease of operation, and compatibility HAMA were 16°C–17°C and 17°C–18°C, respectively,
with a variety of bioinks. Bioinks in the cartridges are based on the sol–gel transition temperatures determined
extruded using either pneumatic pressure or mechanical by rheological analysis. A four-layer circular structure
force to the preset position through a nozzle (Figure 6A). It with an 8-mm diameter was fabricated via layer-by-layer
enables the bioprinting of bioinks with high-cell densities. photocrosslinking in the bioprinting process (Figure 6B
The shear stress generated by high extrusion pressure and C). High-definition images showed that the scaffold
during extrusion-based bioprinting may damage cells, so structure was consistent with the design pattern and that
cell viability needs to be assessed after printing . adECM the scaffolds had a regular porous structure (Figure 6D).
[46]
Volume 9 Issue 4 (2023) 37 https://doi.org/10.18063/ijb.718