Page 145 - IJB-9-6
P. 145
International Journal of Bioprinting 3D printed bioactive dressings for burn wound treatment
(iii) Non-printed hydrogel: Wounds covered with non- viable tissue that appeared black, brown, or gray in color
printed hydrogel and had a dry, leathery texture. The extent of necrotic tissue
(iv) 3D-printed hydrogel: Wounds covered with removal was documented for each sample. The animals
3D-printed hydrogel dressings were euthanized after 4 weeks using a lethal dose of CO .
2
Wound tissue explants were collected and fixed in formalin
(v) Non-printed hydrogel–BBG: Wounds covered with solution overnight for further histology investigation.
non-printed hydrogel–BBG
2.9.2. Wound closure
(vi) 3D-printed hydrogel–BBG: Wounds covered with Wounds were rebandaged and photographed every 7 days
3D-printed hydrogel–BBG dressings to track the wound size, color, edge, re-epithelialization,
The animals were anesthetized using isoflurane. necrotic tissue formation, and secondary trauma caused
After shaving the dorsal area, the skin was cleaned with by dressing removal. A sterile disposable ruler was placed
iodine and then sterilized with alcohol swabs three times. in close proximity to the wound, serving as a scale for
The second-degree burn was made by placing a 100°C measurement purposes. The wound size was quantified by
aluminum bar with a diameter of 20 mm on the dorsal tracing the wound border in each photograph using ImageJ
area for 10 s. After implementation, the wounds were software. The wound closure was calculated as follows:
disinfected by Dermoplast antiseptic spray (Advantice
Health LLC, New Jersey, USA). After applying the (V)
dressings, the wounds were covered with Petrolatum
Gauze and Elastikon bandages (3M, Minnesota, USA). where A is the wound area after wound creation, and
0
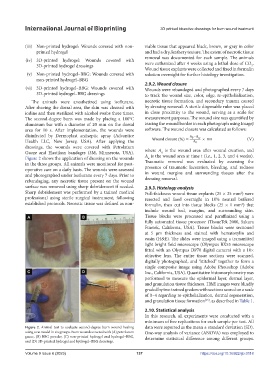
Figure 2 shows the application of dressing on the wounds A is the wound area at time t (i.e., 1, 2, 3, and 4 weeks).
w
in the three groups. All animals were monitored for post‐ Traumatic removal was evaluated by assessing the
operative care on a daily basis. The wounds were assessed presence of traumatic laceration, bleeding, and redness
and photographed under isoflurane every 7 days. Prior to in wound margins and surrounding tissues after the
rebandaging, any necrotic tissue present on the wound dressing removal.
surface was removed using sharp debridement if needed. 2.9.3. Histology analysis
Sharp debridement was performed by a trained medical Full-thickness wound tissue explants (25 × 25 mm ) were
2
professional using sterile surgical instrument, following resected and fixed overnight in 10% neutral buffered
established protocols. Necrotic tissue was defined as non- formalin, then cut into tissue blocks (25 × 1 mm ) that
2
include wound bed, margins, and surrounding skin.
Tissue blocks were processed and paraffinized using a
fully automated tissue processor (TissueTek 2000, Sakura
Finetek, California, USA). Tissue blocks were sectioned
at 5 µm thickness and stained with hematoxylin and
eosin (H&E). The slides were imaged using a transmitted
light bright field microscope (Olympus BX53 microscope
fitted with an Olympus DP70 digital camera) with a 10×
objective lens. The entire tissue sections were scanned,
digitally photographed, and “stitched” together to form a
single composite image using Adobe Photoshop (Adobe
Inc., California, USA). Quantitative histomorphometry was
performed to measure the epidermal layer, dermal layer,
and granulation tissue thickness. H&E images were blindly
graded by two trained graders with sections scored on a scale
of 0–4 regarding re-epithelialization, dermal regeneration,
and granulation tissue formation , as described in Table 1.
[65]
2.10. Statistical analysis
In this research, all experiments were conducted with a
minimum of five replications for each sample per test. All
Figure 2. Animal test to evaluate second-degree burn wound healing data were reported as the mean ± standard deviation (SD).
using a rat model in six groups. Burn wounds covered with (A) petrolatum One-way analysis of variance (ANOVA) was employed to
gauze, (B) BBG powder, (C) non-printed hydrogel and hydrogel–BBG, determine statistical difference among different groups.
and (D) 3D-printed hydrogel and hydrogel–BBG dressings.
Volume 9 Issue 6 (2023) 137 https://doi.org/10.36922/ijb.0118