Page 351 - v11i4
P. 351
International Journal of Bioprinting GradGelMA 3D-bioprinted vascular skin
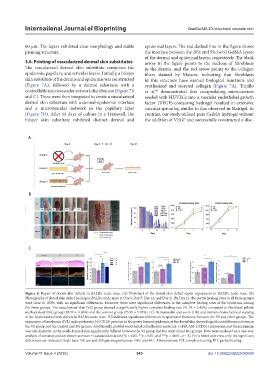
60 μm. The layers exhibited clear morphology and stable epidermal layers. The red dashed line in the figure shows
printing structure. the interface between the 20% and 5% (w/v) GelMA layers
of the dermal and epidermal layers, respectively. The black
3.6. Printing of vascularized dermal skin substitutes arrow in the figure points to the nucleus of fibroblasts
The vascularized dermal skin substitute comprises the in the dermis, and the red arrow points to the collagen
epidermis, papillary, and reticular layers. Initially, a bilayer fibers stained by Masson, indicating that fibroblasts
skin substitute of the dermis and epidermis was constructed in this structure have exerted biological functions and
(Figure 7A), followed by a dermal substitute with a synthesized and secreted collagen (Figure 7A). Trujillo
controllable microvascular network distribution (Figure 7B et al. demonstrated that encapsulating microcarriers
49
and C). These were then integrated to create a vascularized seeded with HUVECs into a vascular endothelial growth
dermal skin substitute with a dermal-epidermal interface factor (VEGF)-containing hydrogel resulted in extensive
and a microvascular network in the papillary layer vascular sprouting similar to that observed in Matrigel. In
(Figure 7D). After 14 days of culture in a Transwell, the contrast, our study utilized pure GelMA hydrogel without
bilayer skin substitute exhibited distinct dermal and the addition of VEGF and successfully constructed a disc-
Figure 8. Repair of dorsal skin defects in BALB/c nude mice. (A) Flowchart of the dorsal skin defect repair experiment in BALB/c nude mice. (B)
Photographs of dorsal skin defect healing in BALB/c nude mice at Day 0, Day 7, Day 14, and Day 21. By Day 21, the partial healing rates in all three groups
were close to 100%, with no significant differences. However, there were significant differences in the complete healing rates of the epidermis among
the three groups. The vascularized skin (VS) group showed a significantly higher complete healing rate (91.78 ± 5.42%) compared to the blank gelatin
methacryloyl (BG) group (85.51 ± 6.96%) and the control group (75.99 ± 5.81%). (C) Hematoxylin and eosin (HE) and immunohistochemical staining
of the healed dorsal skin defects in BALB/c nude mice. HE indicated significant differences in epidermal thickness between the VS and other groups. The
expression of involucrin (IVL) and cytokeratin 10 (CK10) proteins in the newly formed epidermis of the dorsal skin showed significant differences between
the VS group and the control and BG groups. Additionally, platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) expression and the maximum
vascular diameter in the newly formed skin significantly differed between the VS group and the control and BG groups. Data were analyzed via a one-way
analysis of variance and are shown as mean ± standard deviation (*p < 0.05, **p < 0.01, and ***p < 0.001, n = 3). For a better overview, only the significant
differences are indicated. Scale bars: 100 µm and 500 µm; magnification: 100× and 40×. Abbreviations: CH, complete healing; PH, partial healing.
Volume 11 Issue 4 (2025) 343 doi: 10.36922/IJB025090069