Page 363 - v11i4
P. 363
International Journal of Bioprinting Sr-doped printed scaffolds for bone repair
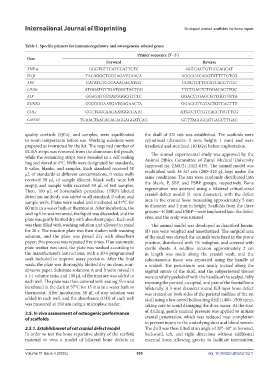
Table 1. Specific primers for immunoregulatory and osteogenesis-related genes
Primer sequence (5’–3’)
Gene
Forward Reverse
TNF-α GGGTGTTCATCCATTCTC GGTCACTGTCCCAGCAT
IL1β TACAGGCTCCGAGATGAACA AGGCCACAGGTATTTTGTCG
ARG CATATCTGCCAAAGACATCG GGTCTCTTCCATCACCTTGC
CD206 ATGGATGTTGATGGCTACTGG TTCTGACTCTGGACACTTGC
ALP GGAGATGGTATGGGCGTCTC GGACCTGAGCGTTGGTGTTA
RUNX2 GCCGGGAATGATGAGAACTA GGACCGTCCACTGTCACTTT
COL1 CGCTGGCAAGAATGGCGATC ATGCCTCTGTCACCTTGTTCG
GAPDH TGAACTAACACAGAGGAGGATCAG GCTTAGGGCATGAGCTTGAC
quality controls (QCs), and samples, were equilibrated the skull of SD rats was established. The scaffolds were
to room temperature before use. Working solutions were cylindrical (diameter: 5 mm; height: 1 mm) and were
prepared as instructed by the kit. The required number of irradiated and sterilized (10 kGy) before implantation.
ELISA strips was removed from the aluminum foil pouch, The animal experimental study was approved by the
while the remaining strips were resealed in a self-sealing Animal Ethics Committee of Zunyi Medical University
bag and stored at 4°C. Wells were designated for standards, (approval no. ZMU21-2412-019). The animal model was
0 value, blanks, and samples. Each standard received 50
μL of standards at different concentrations, 0 value wells established with 36 SD rats (280–320 g), kept under the
received 50 μL of sample diluent, blank wells were left same conditions. The rats were randomly distributed into
empty, and sample wells received 50 μL of test samples. the blank, P, SBP, and PSBP groups, respectively. Bone
Then, 100 μL of horseradish peroxidase (HRP)-labeled regeneration was assessed using a bilateral critical-sized
detection antibody was added to all standard, 0 value, and cranial defect model (5 mm diameter), with the defect
sample wells. Plates were sealed and incubated at 37°C for area in the cranial bone measuring approximately 5 mm
60 min in a water bath or thermostat. After incubation, the in diameter and 2 mm in height. Scaffolds from the three
sealing film was removed, the liquid was discarded, and the groups—P, SBP, and PSBP—were implanted into the defect
plate was gently blotted dry with absorbent paper. Each well sites, and the scalp was sutured.
was then filled with washing solution and allowed to stand The animal model was developed as described herein.
for 20 s. The reaction plate was then shaken with washing SD rats were weighed and anesthetized. The surgical area
solution, and the plate was patted dry with absorbent of the skull was shaved, the animals were fixed in the prone
paper; this process was repeated five times. If an automatic position, disinfected with 1% iodophor, and covered with
plate washer was used, the plate was washed according to sterile sheets. A midline incision approximately 2 cm
the manufacturer’s instructions, with a 30-s programmed in length was made along the cranial vault, and the
soak included to improve assay precision. After the final subcutaneous tissue was separated using the handle of
wash, the plate was thoroughly blotted dry on clean, non- a scalpel. The periosteum was neatly incised along the
abrasive paper. Substrate solutions A and B were mixed in sagittal suture of the skull, and the subperiosteal tissues
a 1:1 volume ratio, and 100 μL of the mixture was added to were carefully peeled off with the handle of the scalpel, fully
each well. The plate was then covered with sealing film and exposing the parietal, occipital, and part of the frontal bone
incubated in the dark at 37°C for 15 min in a water bath or bilaterally. A 5-mm diameter round full-layer bone defect
thermostat. After incubation, 50 μL of stop solution was was created on both sides of the parietal midline of the rat
added to each well, and the absorbance (OD) of each well skull using a low-speed hollow ring drill (1400–1500 rpm),
was measured at 450 nm using a microplate reader. taking care to avoid damaging the dura mater. At the start
2.5. In vivo assessment of osteogenic performance of drilling, gentle manual pressure was applied to initiate
of scaffolds cranial penetration, which was reduced near completion
to prevent injury to the underlying dura and blood vessels.
2.5.1. Establishment of rat cranial defect model The drill was then tilted at an angle of 20°–30° in forward,
In order to test the bone reparative ability of the scaffold backward, left, and right directions without additional
material in vivo, a model of bilateral bone defects in external force, allowing gravity to facilitate intermittent
Volume 11 Issue 4 (2025) 355 doi: 10.36922/IJB025210211