Page 391 - v11i4
P. 391
International Journal of Bioprinting Bioprinted vascular tumor model
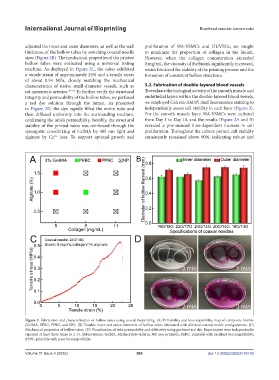
adjusted the inner and outer diameters, as well as the wall proliferation of HA-VSMCs and HUVECs, we sought
thickness, of the hollow tubes by switching coaxial needle to maximize the proportion of collagen in the bioink.
sizes (Figure 2B). The mechanical properties of the printed However, when the collagen concentration exceeded
hollow tubes were evaluated using a universal testing 8 mg/mL, the viscosity of the bioink significantly increased,
machine. As displayed in Figure 2C, the tubes exhibited which hindered the stability of the printing process and the
a tensile strain of approximately 23% and a tensile stress formation of consistent hollow structures.
of about 0.54 MPa, closely matching the mechanical
characteristics of native small-diameter vessels, such as 3.2. Fabrication of double-layered blood vessels
rat mesenteric arteries. 40–42 To further verify the structural To evaluate the biological activity of the smooth muscle and
integrity and permeability of the hollow tubes, we perfused endothelial layers within the double-layered blood vessels,
a red dye solution through the lumen. As presented we employed Calcein-AM/PI dual fluorescence staining to
in Figure 2D, the dye rapidly filled the entire tube and independently assess cell viability in each layer (Figure 3).
then diffused uniformly into the surrounding medium, For the smooth muscle layer, HA-VSMCs were cultured
confirming the tube’s permeability. Notably, the structural from Day 1 to Day 10, and the results (Figure 3A and B)
stability of the printed tubes was reinforced through the revealed a pronounced time-dependent increase in cell
synergistic crosslinking of GelMA by 405 nm light and proliferation. Throughout the culture period, cell viability
alginate by Ca² ions. To support optimal growth and consistently remained above 90%, indicating robust and
+
Figure 2. Fabrication and characterization of hollow tubes using coaxial bioprinting. (A) Printability and biocompatibility map of composite bioinks
(GelMA, PEBC, PPBC, and NP). (B) Tunable inner and outer diameters of hollow tubes fabricated with different coaxial nozzle configurations. (C)
Mechanical properties of hollow tubes. (D) Visualization of tube permeability and diffusivity using perfused red dye. Experiments were independently
repeated at least three times (n ≥ 3). Abbreviations: GelMA, Methacrylate Gelatin; NP, non-printable; PEBC, printable with excellent biocompatibility;
PPBC, printable with poor biocompatibility.
Volume 11 Issue 4 (2025) 383 doi: 10.36922/IJB025180180