Page 392 - v11i4
P. 392
International Journal of Bioprinting Bioprinted vascular tumor model
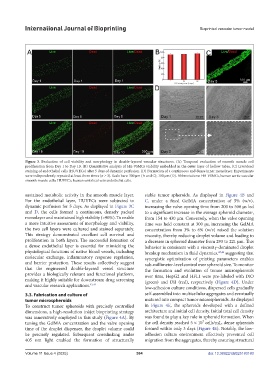
Figure 3. Evaluation of cell viability and morphology in double-layered vascular structures. (A) Temporal evaluation of smooth muscle cell
proliferation from Day 1 to Day 10. (B) Quantitative analysis of HA-VSMCs viability embedded in the outer layer of hollow tubes. (C) Live/dead
staining of endothelial cells (HUVECs) after 5 days of dynamic perfusion. (D) Formation of a continuous and dense inner monolayer. Experiments
were independently repeated at least three times (n ≥ 3). Scale bars: 300 µm (A and C); 100 µm (D). Abbreviations: HA-VSMCs, human aortic vascular
smooth muscle cells; HUVECs, human umbilical vein endothelial cells.
sustained metabolic activity in the smooth muscle layer. stable tumor spheroids. As displayed in Figure 4B and
For the endothelial layer, HUVECs were subjected to C, under a fixed GelMA concentration of 5% (w/v),
dynamic perfusion for 5 days. As displayed in Figure 3C increasing the valve opening time from 200 to 500 μs led
and D, the cells formed a continuous, densely packed to a significant increase in the average spheroid diameter,
monolayer and maintained high viability (>90%). To enable from 154 to 430 μm. Conversely, when the valve opening
a more intuitive assessment of morphology and viability, time was held constant at 300 μs, increasing the GelMA
the two cell layers were cultured and stained separately. concentration from 3% to 6% (w/v) raised the solution
This strategy demonstrated excellent cell survival and viscosity, thereby reducing droplet volume and leading to
proliferation in both layers. The successful formation of a decrease in spheroid diameter from 293 to 221 μm. This
a dense endothelial layer is essential for mimicking the behavior is consistent with a viscosity-dominated droplet
physiological functions of native blood vessels, including breakup mechanism in fluid dynamics, 45,46 suggesting that
molecular exchange, inflammatory response regulation, synergistic optimization of printing parameters enables
and barrier protection. These results collectively suggest sub-millimeter-level control over spheroid size. To monitor
that the engineered double-layered vessel structure the formation and evolution of tumor microspheroids
provides a biologically relevant and functional platform, over time, HepG2 and HFL1 were pre-labeled with DiO
making it highly suitable for downstream drug screening (green) and DiI (red), respectively (Figure 4D). Under
and vascular research applications. 43,44 low-adhesion culture conditions, dispersed cells gradually
3.3. Fabrication and culture of self-assembled into multicellular aggregates and eventually
tumor microspheroids matured into compact tumor microspheroids. As displayed
To construct tumor spheroids with precisely controlled in Figure 4E, the spheroids developed with a defined
dimensions, a high-resolution inkjet bioprinting strategy architecture and initial cell density. Initial total cell density
was innovatively employed in this study (Figure 4A). By was found to play a key role in spheroid formation. When
tuning the GelMA concentration and the valve opening the cell density reached 5 × 10⁷ cells/mL, dense spheroids
time of the droplet dispenser, the droplet volume could formed within only 3 days (Figure 4E). Notably, the low-
be precisely regulated. Subsequent crosslinking under adhesion culture environment effectively prevented cell
405 nm light enabled the formation of structurally migration from the aggregates, thereby ensuring structural
Volume 11 Issue 4 (2025) 384 doi: 10.36922/IJB025180180