Page 59 - JCTR-9-4
P. 59
Li et al. | Journal of Clinical and Translational Research 2023; 9(4): 272-281 275
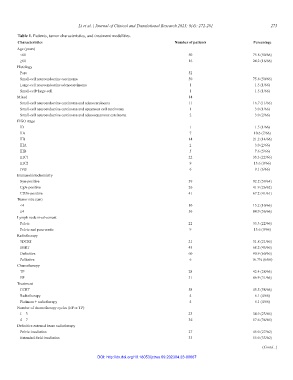
Table 1. Patients, tumor characteristics, and treatment modalities.
Characteristics Number of patients Percentage
Age (years)
<60 50 75.8 (50/66)
≥60 16 24.2 (16/66)
Histology
Pure 52
Small-cell neuroendocrine carcinoma 50 75.8 (50/66)
Large-cell neuroendocrine adenocarcinoma 1 1.5 (1/66)
Small-cell+large-cell 1 1.5 (1/66)
Mixed 14
Small-cell neuroendocrine carcinoma and adenocarcinoma 11 16.7 (11/66)
Small-cell neuroendocrine carcinoma and squamous cell carcinoma 1 3.0 (1/66)
Small-cell neuroendocrine carcinoma and adenosquamous carcinoma 2 3.0 (2/66)
FIGO stage
IB 1 1.5 (1/66)
IIA 7 10.6 (7/66)
IIB 14 21.2 (14/66)
IIIA 2 3.0 (2/66)
IIIB 5 7.6 (5/66)
IIIC1 22 33.3 (22/66)
IIIC2 9 13.6 (9/66)
IVB 6 9.1 (6/66)
Immunohistochemistry
Syn-positive 59 92.2 (59/64)
CgA-positive 26 41.9 (26/62)
CD56-positive 41 67.2 (41/61)
Tumor size (cm)
<4 10 15.2 (10/66)
≥4 56 84.9 (56/66)
Lymph node involvement
Pelvic 22 33.3 (22/66)
Pelvic and para-aortic 9 13.6 (9/66)
Radiotherapy
3DCRT 21 31.8 (21/66)
IMRT 45 68.2 (45/66)
Definitive 60 90.9 (60/66)
Palliative 6 16.7% (6/66)
Chemotherapy
TP 28 42.4 (28/66)
EP 31 46.9 (31/66)
Treatment
CCRT 58 45.5 (58/66)
Radiotherapy 4 6.1 (4/66)
Platinum + radiotherapy 4 6.1 (4/66)
Number of chemotherapy cycles (EP or TP)
1 – 3 23 34.9 (23/66)
4 – 7 36 57.6 (36/66)
Definitive external beam radiotherapy
Pelvic irradiation 27 45.0 (27/60)
Extended-field irradiation 33 55.0 (33/60)
(Contd...)
DOI: http://dx.doi.org/10.18053/jctres.09.202304.23-00067