Page 73 - JCTR-9-4
P. 73
Al-Qahtani et al. | Journal of Clinical and Translational Research 2023; 9(4): 282-289 289
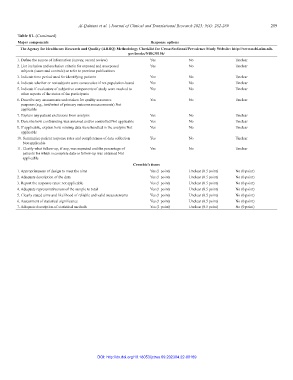
Table S1. (Continued)
Major components Response options
The Agency for Healthcare Research and Quality (AHRQ) Methodology Checklist for Cross‑Sectional/Prevalence Study Website: http://www.ncbi.nlm.nih.
gov/books/NBK35156/
1. Define the source of information (survey, record review) Yes No Unclear
2. List inclusion and exclusion criteria for exposed and unexposed Yes No Unclear
subjects (cases and controls) or refer to previous publications
3. Indicate time period used for identifying patients Yes No Unclear
4. Indicate whether or not subjects were consecutive if not population‑based Yes No Unclear
5. Indicate if evaluators of subjective components of study were masked to Yes No Unclear
other aspects of the status of the participants
6. Describe any assessments undertaken for quality assurance Yes No Unclear
purposes (e.g., test/retest of primary outcome measurements) Not
applicable
7. Explain any patient exclusions from analysis Yes No Unclear
8. Describe how confounding was assessed and/or controlled Not applicable Yes No Unclear
9. If applicable, explain how missing data were handled in the analysis Not Yes No Unclear
applicable
10. Summarize patient response rates and completeness of data collection Yes No Unclear
Not applicable
11. Clarify what follow‑up, if any, was expected and the percentage of Yes No Unclear
patients for which incomplete data or follow‑up was obtained Not
applicable
Crombie’s items
1. Appropriateness of design to meet the aims Yes (1 point) Unclear (0.5 point) No (0 point)
2. Adequate description of the data Yes (1 point) Unclear (0.5 point) No (0 point)
3. Report the response rates: not applicable Yes (1 point) Unclear (0.5 point) No (0 point)
4. Adequate representativeness of the sample to total Yes (1 point) Unclear (0.5 point) No (0 point)
5. Clearly stated aims and likelihood of reliable and valid measurements Yes (1 point) Unclear (0.5 point) No (0 point)
6. Assessment of statistical significance Yes (1 point) Unclear (0.5 point) No (0 point)
7. Adequate description of statistical methods Yes (1 point) Unclear (0.5 point) No (0 point)
DOI: http://dx.doi.org/10.18053/jctres.09.202304.22-00189