Page 52 - MSAM-3-2
P. 52
Materials Science in Additive Manufacturing Heat treatment on bimetallic parts
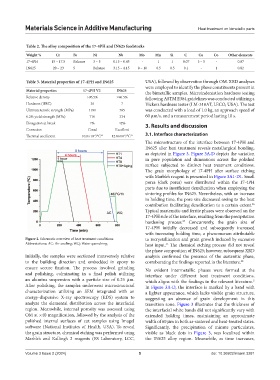
Table 2. The alloy composition of the 17‑4PH and IN625 feedstocks
Weight % Cr Fe Ni Nb Mo Mn Si C Cu Co Other elements
17-4PH 15 – 17.5 Balance 3 – 5 0.15 – 0.45 - 1 1 0.07 3 – 5 - 0.07
IN625 20 – 23 5 Balance 3.15 – 4.15 8 – 10 0.5 0.5 0.1 - 1 0.82
Table 3. Material properties of 17‑4PH and IN625 USA), followed by observation through OM. XRD analyses
were employed to identify the phase constituents present in
Material properties 17‑4PH V2 IN625 the bimetallic samples. Microindentation hardness testing
Relative density >96.5% >96.5% following ASTM E384 guidelines was conducted utilizing a
Hardness (HRC) 36 7 Vickers hardness tester (LM-310AT, LECO, USA). The test
Ultimate tensile strength (MPa) 1180 765 was conducted with a load of 1.0 kg, an approach speed of
0.2% yield strength (MPa) 710 334 60 μm/s, and a measurement period lasting 10 s.
Elongation at break 7% 42% 3. Results and discussion
Corrosion Good Excellent
3.1. Interface characterization
Thermal coefficient 10.8×10 /°C 16 12.8×10 /°C 17
-6
-6
The microstructure of the interface between 17-4PH and
IN625 after heat treatment reveals metallurgical bonding,
as depicted in Figure 3. Figure 3A-D depicts the variation
in pore population and dimensions across the polished
surface subjected to distinct heat treatment conditions.
The grain morphology of 17-4PH after surface etching
with Marble’s reagent is presented in Figure 3A1-D1. Small
pores (dark pores) were distributed within the 17-4PH
parts due to insufficient densification when employing the
sintering profiles for IN625. Nevertheless, with an increase
in holding time, the pore size decreased owing to the heat
contribution facilitating densification to a certain extent.
36
Typical martensitic and ferrite phases were observed on the
17-4PH side of the interface, resulting from the precipitation
hardening process. Concurrently, the grain size of
37
17-4PH initially decreased and subsequently increased
with increasing holding time, a phenomenon attributable
Figure 2. Schematic overview of heat treatment conditions. to recrystallization and grain growth induced by excessive
Abbreviations: AC: Air cooling; WQ: Water quenching. heat input. The chemical etching process did not reveal
32
the phase composition of IN625; however, subsequent XRD
Initially, the samples were sectioned transversely relative analysis confirmed the presence of the austenitic phase,
to the building direction and embedded in epoxy to corroborating the findings reported in the literature. 38
ensure secure fixation. The process involved grinding No evident intermetallic phases were formed at the
and polishing, culminating in a final polish utilizing interface under different heat treatment conditions,
an alumina suspension with a particle size of 0.25 μm. which aligns with the findings in the relevant literature.
5
After polishing, the samples underwent microstructural In Figure 3A-D, the interface is marked by a band with
characterization utilizing an SEM integrated with an a lighter appearance, which lacks visible grain structure,
energy-dispersive X-ray spectroscopy (EDS) system to suggesting an absence of grain development in this
analyze the elemental distribution across the interfacial transition zone. Figure 3 illustrates that the thickness of
region. Meanwhile, internal porosity was assessed using the interfacial white bands did not significantly vary with
OM at ×40 magnification, followed by the analysis of the extended holding times, maintaining an approximate
polished internal surfaces of cut samples using ImageJ width of 60 μm in both as-sintered and heat-treated states.
software (National Institutes of Health, USA). To reveal Significantly, the precipitation of minute particulates,
the grain structure, chemical etching was performed using visible as black dots in Figure 3, was localized within
Marble’s and Kalling’s 2 reagents (ES Laboratory, LCC, the IN625 alloy region. Meanwhile, as time increases,
Volume 3 Issue 2 (2024) 5 doi: 10.36922/msam.3281