Page 89 - MSAM-3-2
P. 89
Materials Science in Additive Manufacturing Sustainable resin for coral restoration
conditions to evaluate the viability of HDFs. This kit
enables the discrimination of live cells, which emit vibrant
green fluorescence on interaction with calcein (excitation/
emission ~494/517 nm). Simultaneously, ethidium
homodimer-1 (EthD-1; excitation/emission ~528/617 nm)
binds to the DNA of the dead cells with compromised cell
membranes and releases red fluorescent light. 37
The procedure involved replacing the culture media
with a mixture of EthD-1 and calcein diluted in 1× PBS.
This mixture was then incubated with the cells for 25 min
at room temperature. Subsequently, a fluorescence
microscope (EVOS M7000 Imaging System) was utilized
to capture images of the cells and analyze their viability.
In addition, to evaluate HDF proliferation under four
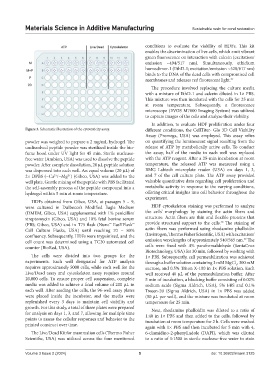
Figure 3. Schematic illustration of the cytotoxicity assay. different conditions, the CellTiter- Glo 3D Cell Viability
Assay (Promega, USA) was employed. This assay relies
powder was weighed to prepare a 2 mg/mL hydrogel. The on quantifying the luminescent signal resulting from the
undissolved peptide powder was sterilized inside the bio- release of ATP by metabolically active cells. To conduct
fume hood under UV light for 45 min. Sterile nuclease- the assay, half of the media in each well was replaced
free water (Ambion, USA) was used to dissolve the peptide with the ATP reagent. After a 25-min incubation at room
powder. After complete dissolution, 20 µL peptide solution temperature, the released ATP was measured using a
was dispensed into each well. An equal volume (20 µL) of BMG Labtech microplate reader (USA) on days 1, 3,
2× DPBS (−Ca /−Mg ) (Gibco, USA) was added to the and 7 of the cell culture plate. The ATP assay provided
2+
2+
well plate. Gentle mixing of the peptide with PBS facilitated valuable quantitative data regarding cell proliferation and
the self-assembly process of the peptide compound into a metabolic activity in response to the varying conditions,
hydrogel within 5 min at room temperature. offering critical insights into cell behavior throughout the
experiment.
HDFs obtained from Gibco, USA, at passages 5 – 9,
were cultured in Dulbecco’s Modified Eagle Medium HDF cytoskeleton staining was performed to analyze
(DMEM, Gibco, USA) supplemented with 1% penicillin/ the cells’ morphology by staining the actin fibers and
streptomycin (Gibco, USA) and 10% fetal bovine serum structure. Actin fibers are thin and flexible proteins that
38
(FBS; Gibco, USA) and in T75 flask (Nunc™ EasYFlask™ provide structural support to the cells. The staining of
Cell Culture Flasks, USA) until reaching 70 − 80% actin fibers was performed using rhodamine phalloidin
confluency. Subsequently, HDFs were trypsinized, and the (Invitrogen, Thermo Fisher Scientific, USA) with excitation/
39
cell count was determined using a TC20 automated cell emission wavelengths of approximately 540/565 nm. The
counter (BioRad, USA). cells were fixed with 4% paraformaldehyde (SantaCruz
Biotechnology, USA) for 30 min, followed by washing with
The cells were divided into two groups for the 1× PBS. Subsequently, cell permeabilization was achieved
experiments. Each well designated for ATP analysis through a buffer solution containing 3 mM MgCl , 300 mM
2
requires approximately 5000 cells, while each well for the sucrose, and 0.5% Triton X-100 in 1× PBS solution. Each
Live/Dead assay and cytoskeleton assay requires around well received 40 µL of the permeabilization buffer. After
20,000 cells. To ensure proper cell suspension, complete 5 min of incubation, a blocking buffer consisting of 0.02%
media was added to achieve a final volume of 200 µL in sodium azide (Sigma Aldrich, USA), 5% FBS and 0.1%
each well. After seeding the cells, the 96-well assay plates Tween-20 (Sigma Aldrich, USA) in 1× PBS was added
were placed inside the incubator, and the media were (50 µL per well), and the mixture was incubated at room
replenished every 3 days to maintain cell viability and temperature for 25 min.
growth. For this study, a total of three plates were prepared Next, rhodamine phalloidin was diluted to a ratio of
for analysis on days 1, 3, and 7, allowing for multiple time 1:40 in 1× PBS and then added to the cells, followed by
points to assess the cellular responses and behavior to the incubation at room temperature for 2 h. Cells were washed
printed construct over time.
again with 1× PBS and then incubated for 5 min with 4,
The Live/Dead Kit for mammalian cells (Thermo Fisher 6-diamidino-2-phenylindole (DAPI), which was diluted
Scientific, USA) was utilized across the four mentioned to a ratio of 1:1500 in sterile nuclease-free water to stain
Volume 3 Issue 2 (2024) 5 doi: 10.36922/msam.3125