Page 96 - ARNM-2-3
P. 96
Advances in Radiotherapy
& Nuclear Medicine Radiotherapy with neutron/gamma tubes
of these X-ray photons is the acceleration voltage of the ions are then accelerated to the target electrode where the
electron beam. Since most Bremsstrahlung photons p- F gammas are produced.
19
have energies much lower than the end-point energy, The p- F reaction has a high resonance cross-
8
19
they may not be useful for the intended application and section of 160 mb at 340 keV proton energy. It also has
contribute significant doses to the surroundings while two resonances at ~900 keV proton energy. To take
producing unwanted background radiation. The thick advantage of the high resonance cross-section for gamma
shielding needed to remove these lower energy photons production, the H ions need to be accelerated to energies
−
results in an accelerator system that is generally quite of 1 MeV or higher. Thin layers of CaF or MgF can serve
large and not suitable for some applications. High-energy as the target materials. The thick-target gamma yields
2
2
gamma photons (or gamma rays) can be produced by the have been measured for incident proton energies between
decay of some radioisotopes. For example, cesium-137 17,18
emits 662-keV gamma-rays and cobalt-60 emits a pair of 1.5 and 4 MeV. From the result of these measurements,
1.13 and 1.33 MeV gammas. Due to their high activities, the thick-target gamma yield for a 1 MeV proton beam is
estimated to be about 2 × 10 γ/mA/s. By operating the
10
these radioisotopes are very compact and intense gamma mini gamma-ray tube at 10% DF, the average gamma-ray
sources. The drawbacks are that they are always “on,” yield is 2 × 10 γ/mA/s. Assuming the tumor is located
9
require thickly shielded containers for safety purposes,
and are regulated radioactive materials. The Gamma 2 cm from the surface, the gamma flux at the tumor site
7
2
Knife device incorporates 201 cobalt-60 sources housed will become 4 × 10 γ/mA/cm /s. This flux can be greatly
in the central body of the unit. These sources produce increased by employing the multiple tube arrangement
201 collimated beams directed to a single focal point as illustrated in Figure 7. With seven mini tubes (each
(machine isocenter). A newly loaded Gamma Knife has a operating at 1 MV, 10 mA, and 10% DF), the total gamma
9
2
total activity of the order of 220 TBq (6000 Ci) providing a flux at the tumor site can exceed 2.8 × 10 γ/cm /s. This
gamma flux between 2.8 to 7 × 10 γ/cm /s at the isocenter. estimated gamma flux at the tumor site is approximately
8
9
2
In its main application for the treatment of brain cancer, the same as that of the Gamma Knife.
the Gamma Knife provides a typical dose rate on the order There are several advantages in using multiple mini
of 1 – 2 Gray/min at the tumor site. 8 tubes for brain tumor treatment. First, the gamma beams
Alternatively, megavolt-energy gamma rays can also can be turned off when they are not in use. Second, the
19
be produced by bombarding a target with accelerated ions energy of the p- F gamma photon is higher than that of
(e.g., protons or deuterons) to create nuclear reactions in the Gamma Knife. They have better tumor penetration
the target. Of all these reactions, the F(p,αγ) O reaction ability. Therefore, cancer treatment is more effective for the
16
19
8
can produce high-energy gamma-rays of 6.1, 6.9, and 7.1 gamma tube system. Third, since the gamma beams are
MeV, which are suitable for radiotherapy applications. operating in short pulses, FLASH photon therapy can be
8
10
Using the p- F reaction, coupled with a mini gamma- performed in the mini gamma tube system. Fourth, the
19
ray generator, one can produce a useful gamma yield and machine and maintenance cost for the single or multiple
energy for the radiotherapy treatment of cancer. The mini gamma tube system is much lower than that of the Gamma
gamma-ray tube has a similar design and operation as the Knife. Fifth, the gamma tubes can be mounted on a robotic
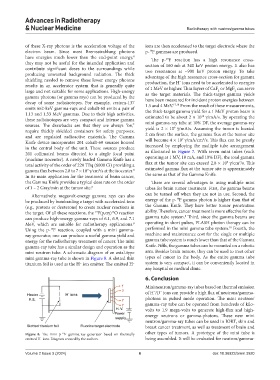
mini neutron tube. A schematic diagram of an axial-type arm. Besides brain tumors, they can be used to treat other
mini gamma-ray tube is shown in Figure 8. A slotted thin types of cancer in the body. As the entire gamma tube
titanium foil is used as the H ion emitter. The emitted H system is very compact, it can be conveniently located in
−
−
any hospital or medical clinic.
6. Conclusion
Mini neutron/gamma-ray tubes based on thermal emission
of H /D ions can provide a high flux of neutrons/gamma-
−
−
photons in pulsed mode operation. The mini neutron/
gamma-ray tube can be operated from hundreds of kilo-
volts to 1.9 mega-volts to generate high-flux and high-
energy neutrons or gamma-photons. These new mini
neutron/gamma-ray tubes can be used in IORT, skin and
breast cancer treatment, as well as treatment of brain and
Figure 8. The mini p- F gamma-ray generator based on thermally other types of tumors. A prototype of the mini tube is
19
emitted H ions. Diagram created by the authors. being assembled. It will be evaluated for neutron/gamma-
−
Volume 2 Issue 3 (2024) 6 doi: 10.36922/arnm.3920