Page 184 - EJMO-9-3
P. 184
Eurasian Journal of
Medicine and Oncology Research on DSY in treating gastritis
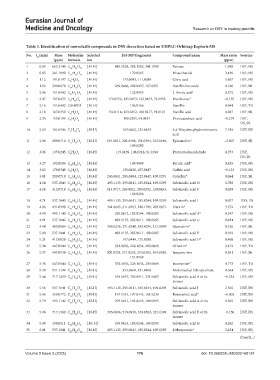
Table 3. Identification of nonvolatile compounds in DSY decoction based on UHPLC‑Orbitrap Exploris MS
No. t (min) Mass Molecular Selected ESI‑MS fragments Compound name Mass error Sources
2
R
(ppm) formula ion (ppm)
1 0.83 665.2148 C H O 21 [M-H] - 485.1528, 383.1202, 341.1098 Tetrose 1.948 DSY, DS
42
24
2 0.85 341.1090 C H O 11 [M-H] - 179.0565 Disaccharide 3.436 DSY, DS
22
12
3 1.12 191.0197 C H O [M-H] - 173.0093, 111.0088 Citric acid 5.607 DSY, DS
6 8 7
4 1.92 329.0879 C H O [M-H] - 269.0666, 209.0457, 167.0351 Vanillin hexoside 0.166 DSY, SR
14 18 9
5 2.06 151.0402 C H O [M-H] - 123.0453 2-Anisic acid 8.272 DSY, DS
8 8 3
6 2.07 197.0455 C H O 5 [M-H] - 179.0352, 135.0453, 123.0453, 72.9932 Danshensu* −0.135 DSY, DS
10
9
7 2.16 151.0402 C8H8O3 [M-H] - 136.0166 Vanillin 0.944 DSY, TX
8 2.18 167.0350 C H O [M-H] - 152.0114, 123.0452, 108.0217, 91.0191 Vanillic acid 0.287 DSY, SR
8 8 4
9 2.35 153.0193 C H O [M-H] - 109.0295, 91.0191 Protocatechuic acid −0.274 DSY,
7 6 4
DS, SR
10 2.63 181.0506 C H O [M-H] - 163.0402, 135.0453 3,4-Dihydroxyhydrocinnamic 7.538 DSY, DS
9 10 4
acid
11 3.04 289.0710 C H O [M-H] - 245.0811, 205.0498, 151.0394, 123.0448, Epicatechin* −2.807 DSY, SR
15 14 6
109.0292
12 3.06 137.0245 C H O 3 [M-H] - 119.0139, 108.0218, 91.0190 Protocatechualdehyde 0.273 DSY,
6
7
DS, SR
13 3.27 193.0506 C H O [M-H] - 149.0608 Ferulic acid* 0.425 DSY, DS
10 10 4
14 3.63 179.0349 C H O [M-H] - 135.0436, 107.0487 Caffeic acid −0.123 DSY, DS
9 8 4
15 3.81 289.0718 C H O [M-H] - 245.0811, 205.0494, 123.0447, 109.0291 Catechin* 0.064 DSY, SR
15 14 6
16 4.54 537.1043 C H O [M-H] - 493.1135, 295.0611, 185.0244, 109.0295 Salvianolic acid H 2.788 DSY, DS
27 22 12
17 4.58 313.0718 C H O 6 [M-H] - 313.0717, 269.0821, 203.0352, 159.0453, Salvianolic acid F 0.059 DSY, DS
17
14
109.0296
18 4.71 537.1043 C H O 12 [M-H] - 493.1135, 295.0611, 185.0244, 109.0295 Salvianolic acid I 0.057 DSY, DS
27
22
19 4.86 431.0999 C H O [M-H] - 341.0645, 311.0563, 228.1595, 203.0871 Vitexin* 3.572 DSY, TX
21 20 10
20 4.90 493.1143 C H O [M-H] - 295.0612, 185.0244, 109.0295 Salvianolic acid A* 0.547 DSY, DS
26 22 10
21 4.91 537.1042 C H O [M-H] - 493.1135, 295.0611, 109.0295 Salvianolic acid U 0.634 DSY, DS
27 22 12
22 4.94 463.0884 C H O 12 [M-H] - 300.0276, 271.0249, 243.0295, 151.0039 Quercitrin* 0.326 DSY, SR
21
20
23 5.00 537.1041 C H O 12 [M-H] - 493.1135, 295.0611, 109.0295 Salvianolic acid T 0.392 DSY, DS
22
27
24 5.21 417.0829 C H O [M-H] - 197.0444, 175.0388 Salvianolic acid D* 0.408 DSY, DS
20 18 10
25 5.34 447.0944 C H O [M-H] - 332.0856, 228.1654, 203.0869 Orientin* 2.472 DSY, TX
21 20 11
26 5.37 447.0939 C H O [M-H] - 300.0278, 271.0252, 255.0303, 243.0298, Isoquercitrin 0.281 DSY, SR
21 20 11
151.0039
27 5.38 447.0944 C H O [M-H] - 332.0856, 228.1654, 203.0869 Isoorientin* 0.773 DSY, TX
21 20 11
28 5.39 551.1198 C H O 12 [M-H] - 353.0668, 321.0405 Monomethyl lithospermate 0.564 DSY, DS
28
24
29 5.44 717.1459 C H O 16 [M-H] - 519.0937, 339.0511, 321.0405 Salvianolic acid B or its −0.234 DSY, DS
36
30
isomer
30 5.56 537.1041 C H O [M-H] - 493.1135, 295.0611, 185.0244, 109.0295 Salvianolic acid J 2.565 DSY, DS
27 22 12
31 5.66 359.0772 C H O [M-H] - 197.0444, 197.0444, 161.0230 Rosmarinic acid* −0.002 DSY, DS
18 16 8
32 5.79 493.1142 C H O [M-H] - 295.0611, 185.0243, 109.0295 Salvianolic acid A or its 0.365 DSY, DS
26 22 10
isomer
33 5.98 717.1460 C H O 16 [M-H] - 295.0606, 519.0930, 339.0503, 321.0399 Salvianolic acid B or its −0.150 DSY, DS
36
30
isomer
34 5.99 339.0511 C H O 7 [M+H] - 295.0615, 185.0244, 109.0295 Salvianolic acid G 0.262 DSY, DS
12
18
35 6.01 537.1041 C H O [M-H] - 493.1135, 295.0611, 185.0244, 109.0295 Lithospermic* 2.434 DSY, DS
27 22 12
(Cont’d...)
Volume 9 Issue 3 (2025) 176 doi: 10.36922/EJMO025160124