Page 185 - EJMO-9-3
P. 185
Eurasian Journal of
Medicine and Oncology Research on DSY in treating gastritis
Table 3. (Continued)
No. t (min) Mass Molecular Selected ESI‑MS fragments Compound name Mass error Sources
2
R
(ppm) formula ion (ppm)
36 6.06 431.0999 C H O [M-H] - 341.0645, 311.0563, 228.1595, 203.0871 Isovitexin* 3.572 DSY, TX
21 20 10
37 6.07 493.1142 C H O [M-H] - 295.0612, 185.0244, 109.0295 Salvianolic acid A or its isomer 0.304 DSY, DS
26 22 10
38 6.21 717.1459 C H O [M-H] - 519.0937, 339.0511, 321.0405 Salvianolic acid E 1.295 DSY, DS
36 30 16
39 6.33 717.1462 C H O 16 [M-H] - 519.0937, 339.0511, 321.0405 Salvianolic acid B* 0.184 DSY, DS
36
30
40 6.64 493.1141 C H O 10 [M-H] - 295.0612, 185.0244, 109.0295 Salvianolic acid A or its 0.243 DSY, DS
26
22
isomer
41 7.16 493.1142 C H O [M-H] - 295.0611, 185.0243, 109.0295 Salvianolic acid A or its 0.487 DSY, DS
26 22 10
isomer
42 7.50 491.0986 C H O [M-H] - 293.0455, 265.0506, 197.0455 Isosalvianolic acid C* 0.468 DSY, DS
26 20 10
43 8.65 491.0986 C H O [M-H] - 293.0455, 249.0555, 135.0451 Salvianolic acid C 0.529 DSY, DS
26 20 10
44 10.57 311.1277 C H O [M+H] - 267.1377, 203.0853 Tanshinaldehyde -3.929 DSY, DS
19 18 4
45 10.80 327.1238 C H O 5 [M-H] - 283.1340, 239.1441 1S-hydroxy-anhydride of 0.040 DSY, DS
20
19
16Rcryptotanshinone
46 10.83 311.1277 C H O 4 [M+H] - 293.1191, 275.1058, 203.0852 Tanshinone IIB −0.307 DSY, DS
19
18
47 10.94 309.1119 C H O [M+H] - 265.1223, 223.0755, 203.0854 Salvianolic aldehyde −0.438 DSY, DS
19 16 4
48 11.03 313.1445 C H O [M-H] - 269.1543, 213.0914 Neocryptotanshinone* −0.008 DSY, DS
19 22 4
49 11.04 297.1484 C H O [M+H] - 253.1586, 203.0853 Isocryptotanshinone −0.674 DSY, DS
19 20 3
50 11.23 279.1014 C H O 3 [M+H] - 261.0907, 203.0852 Dihydrotanshinone I* −0.469 DSY, DS
18
14
51 11.51 295.1327 C H O 3 [M+H] - 267.1379, 249.1266, 203.0853 Tanshinone IIA* −4.431 DSY, DS
18
19
52 11.79 297.1482 C H O [M+H] - 279.1378, 203.0852 Cryptotanshinone* −4.704 DSY, DS
19 20 3
Note: *Compared with reference compounds.
Abbreviations: DS: Salvia miltiorrhiza Bge.; DSY: Dan-Shen-Yin; ESI-MS2: Electrospray ionization tandem mass spectrometry; MS: Mass spectrometry;
SR: Amomum villosum Lour.; TX: Santalum album L.; t : Retention time; UHPLC: Ultra-high performance liquid chromatography.
R
A B C
D E
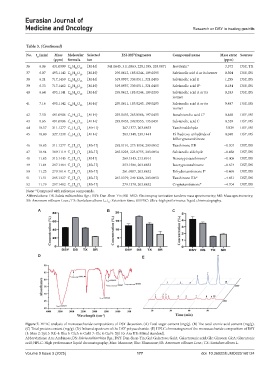
Figure 7. HPLC analysis of monosaccharide compositions of DSY decoction. (A) Total sugar content (mg/g). (B) The total uronic acid content (mg/g).
(C) Total protein content (mg/g). (D) Infrared spectrum of the DSY polysaccharide. (E) HPLC chromatogram of the monosaccharide composition of DSY
(1: Man 2: Lyx 3: Rib 4: Rha 5: GlcA 6: GalA 7: Glc 8: Gal 9: Xyl 10: Ara HB: Mixed standard).
Abbreviations: Ara: Arabinose; DS: Salvia miltiorrhiza Bge.; DSY: Dan-Shen-Yin; Gal: Galactose; GalA: Galacturonic acid; Glc: Glucose; GlcA: Glucuronic
acid; HPLC: High performance liquid chromatography; Man: Mannose; Rha: Rhamnose; SR: Amomum villosum Lour.; TX: Santalum album L.
Volume 9 Issue 3 (2025) 177 doi: 10.36922/EJMO025160124