Page 32 - ESAM-1-4
P. 32
Engineering Science in
Additive Manufacturing Machine learning for biomedical metal AM
Regarding phase composition and morphology load-bearing implants where modulus matching with
prediction, Zhang et al. synthesized 144 sets of Ti– bone tissue is crucial to prevent stress shielding. ML
78
xAl–yV alloy samples through DED and utilized a surrogate models enable predictive mechanical property
backpropagation neural network to establish a mapping design by establishing direct correlations between process
from composition to microstructure and mechanical parameters, heat treatment conditions, and resulting
properties. The models successfully predicted the volume mechanical performance.
fraction of α-phase and the average width of α-laths, In a study on DED titanium alloys, Chi et al. employed
80
achieving high prediction accuracy with R² values an XGBoost model to establish relationships between
exceeding 0.96. This direct composition–microstructure
mapping provides a powerful high-throughput screening process parameters, utilizing laser power, scanning rate,
tool for rapidly designing novel biomedical titanium and heat treatment conditions as inputs to accurately
alloys with tailored microstructures, such as low-modulus predict the ultimate tensile strength (UTS) and elongation
β-Ti alloys. Furthermore, unsupervised deep learning of the material. Ti-17 alloy specimens fabricated under the
has revolutionized microstructure characterization. guidance of the model exhibited mechanical properties
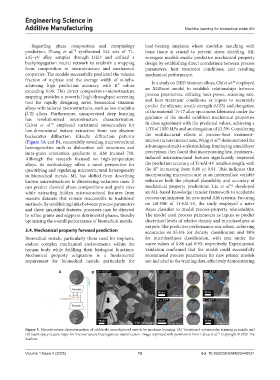
Calvat et al. employed variational autoencoders for in close agreement with the predicted values, achieving a
79
low-dimensional feature extraction from raw electron UTS of 1050 MPa and an elongation of 12.5%. Considering
backscatter diffraction Kikuchi diffraction patterns the multifactorial effects of process–heat treatment–
81
(Figure 5A and B), successfully revealing microstructural microstructure interactions, Wang et al. demonstrated the
heterogeneities such as dislocation cell structures and advantages of multi-scale modeling. Employing a multilayer
intra-grain orientation gradients in AM Inconel 718. perceptron, they found that incorporating heat treatment–
Although the research focused on high-temperature induced microstructural features significantly improved
alloys, its methodology offers a novel perspective for the prediction accuracy of Ti-6Al-4V tensile strength, with
quantifying and regulating microstructural heterogeneity the R² increasing from 0.80 to 0.91. This indicates that
in biomedical metals. ML has shifted from describing incorporating microstructure as an intermediate variable
known microstructures to discovering unknown ones. It enhances both the physical plausibility and accuracy of
82
can predict classical phase compositions and grain sizes mechanical property predictions. Liu et al. developed
while extracting hidden microstructural features from an ML-based knowledge transfer framework to accelerate
massive datasets that remain inaccessible to traditional process optimization for new metal AM systems. Focusing
methods. By establishing links between process parameters on LB-PBF of Ti-6Al-4V, the study employed a naïve
and these quantified features, processes may be directed Bayes classifier to model process-property relationships.
to refine grains and suppress detrimental phases, thereby The model used process parameters as inputs to predict
optimizing the overall performance of biomedical metals. discretized levels of relative density and microhardness as
outputs. The predictive performance was robust, achieving
2.4. Mechanical property forward prediction accuracies of 85.6% for density classification and 88%
Biomedical metals, particularly those used for implants, for microhardness classification, with area under the
endure complex mechanical environments within the curve values of 0.88 and 0.93, respectively. Experimental
human body while fulfilling their biological functions. validation confirmed that the model could successfully
Mechanical property adaptation is a fundamental recommend process parameters for new printer models
requirement for biomedical metals, particularly for not included in the training data, effectively demonstrating
A B
Figure 5. Microstructure characterization of additively manufactured metals by machine learning. (A) Variational autoencoder training principle and
(B) latent space feature maps for microstructure heterogeneity identification. Image reprinted with permission from Calvat et al. Copyright © 2025 The
79
Authors.
Volume 1 Issue 4 (2025) 10 doi: 10.36922/ESAM025440031