Page 162 - IJB-10-1
P. 162
International Journal of Bioprinting 3D-printed micro-perfused culture device
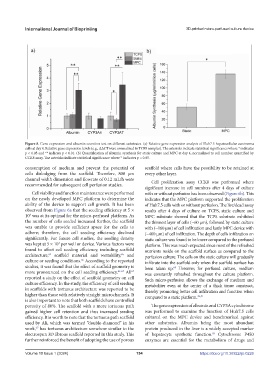
Figure 8. Gene expression and albumin secretion test on different substrates. (a) Relative gene expression analysis of Huh7.5 hepatocellular carcinoma
cells at day 4. Relative gene expression levels (e.g., ∆∆CTwere normalized to TCPS samples). The asterisks indicate statistical significance where * indicates
p < 0.05 and ** indicate p < 0.01. (b) Quantification of albumin synthesis for static culture and MPC at day 4, normalized to cell number quantified by
CCK8 assay. The asterisks indicate statistical significance where * indicates p < 0.05.
consumption of medium and prevent the potential of scaffold where cells have the possibility to be retained at
cells dislodging from the scaffold. Therefore, 800 µm every other layer.
channel width dimension and flowrate of 0.12 mL/h were Cell proliferation assay CCK8 was performed where
recommended for subsequent cell perfusion studies.
significant increase in cell numbers after 4 days of culture
Cell viability and function maintenance were performed with or without perfusion has been observed (Figure 6b). This
on the newly developed MPC platform to determine the indicates that the MPC platform supported the proliferation
ability of the device to support cell growth. It has been of Huh7.5 cells with or without perfusion. The live/dead assay
observed from Figure 6a that the seeding efficiency at 5 × results after 4 days of culture on TCPS, static culture and
4
10 was at its optimal for the micro-perfused platform. As MPC substrate showed that the TCPS substrate exhibited
the number of cells seeded increased further, the scaffold the thinnest layer of cells (~60 µm), followed by static culture
was unable to provide sufficient space for the cells to with (~160 µm) of cell infiltration and lastly MPC device with
adhere; therefore, the cell seeding efficiency declined (~400 µm) of cell infiltration. The depth of cells infiltration on
significantly. For future cell studies, the seeding density static culture was found to be lower compared to the perfused
4
was kept at 5 × 10 per well or device. Various factors were platform. This was much expected since most of the refreshed
found to affect cell seeding efficiency, including scaffold nutrients reside on the scaffold surface as compared to the
65
64
architecture, scaffold material and wettability, and perfusion culture. The cells on the static culture will gradually
66
culture or seeding conditions. According to the reported infiltrate into the scaffold only when the scaffold surface has
studies, it was found that the effect of scaffold geometry is been taken up. However, for perfused culture, medium
68
67
more pronounced on the cell seeding efficiency. 64-67 Ali was constantly refreshed throughout the culture platform.
reported a study on the effect of scaffold geometry on cell Such micro-perfusion allows the exchange of medium and
culture efficiency. In the study, the efficiency of cell seeding metabolites even at the center of a thick tissue construct,
in scaffolds with tortuous architecture was reported to be thereby promoting better cell infiltration and function when
higher than those with relatively straight microchannels. It compared to a static platform. 38,43
is also important to note that both scaffolds have controlled
porosity of 80%. The scaffold with a more tortuous path The gene expression of albumin and CYP3A cytochrome
gained higher cell retention and thus increased seeding was performed to examine the function of Huh7.5 cells
efficiency. It is worth to note that the tortuous path scaffold cultured on the MPC device and benchmarked against
used by Ali, which was termed “double-diamond” in his other substrates. Albumin being the most abundant
work, has tortuous architecture somehow similar to the protein produced in the liver is a widely accepted marker
67
electrospun 3D fibrous scaffold reported in this study. This of hepatocyte synthetic function. Cytochrome P450
69
further reinforced the benefit of adopting the use of porous enzymes are essential for the metabolism of drugs and
Volume 10 Issue 1 (2024) 154 https://doi.org/10.36922/ijb.0226