Page 160 - IJB-10-1
P. 160
International Journal of Bioprinting 3D-printed micro-perfused culture device
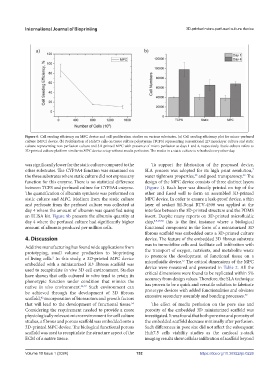
Figure 6. Cell seeding efficiency on MPC device and cell proliferation studies on various substrates. (a) Cell seeding efficiency plot for micro-perfused
culture (MPC) device. (b) Proliferation of Huh7.5 cells on tissue culture polystyrene (TCPS) representing conventional 2D monolayer culture and static
culture representing non-perfusion culture and 3D-printed MPC with presence of micro-perfusion at days 1 and 4, respectively. Static culture refers to
3D-printed culture platform similar to MPC device setup without media perfusion. The media in a static culture is refreshed every other day.
was significantly lower for the static culture compared to the To support the fabrication of the proposed device,
7
other substrates. The CYP3A4 function was examined on SLA process was adopted for its high print resolution,
58
52
the three substrates where static culture did not express any water tightness properties, and good transparency. The
function for this enzyme. There is no statistical difference design of the MPC device consists of three distinct layers
between TCPS and perfused culture for CYP3A4 enzyme. (Figure 1). Each layer was directly printed on top of the
The quantification of albumin synthesis was performed on other and fused well to form an assembled 3D-printed
static culture and MPC. Medium from the static culture MPC device. In order to ensure a leak-proof device, a thin
and perfusate from the perfused culture was collected at layer of sealant Sil-Bond RTV-4500 was applied at the
day 4 where the amount of albumin was quantified using interface between the 3D-printed structure and the PDMS
an ELISA kit. Figure 8b presents the albumin quantity at insert. Despite many reports on 3D-printed microfluidic
day 4 where the perfused culture had significantly higher chip, 8,9,19,58 this is the first instance where a biological
amount of albumin produced per million cells. functional component in the form of a miniaturized 3D
fibrous scaffold was embedded onto a 3D-printed culture
4. Discussion device. The feature of the embedded 3D fibrous substrate
was to immobilize cells and facilitate cell infiltration with
Additive manufacturing has found wide applications from
prototyping, small volume production to bioprinting the transport of oxygen, nutrients, and metabolite waste
to promote the development of functional tissue on a
of living cells. In this study, a 3D-printed MPC device microfluidic device. The critical dimensions of the MPC
5
57
embedded with a miniaturized 3D fibrous scaffold was
used to recapitulate in vivo 3D cell environment. Studies device were measured and presented in Table 2. All the
have shown that cells cultured in vitro tend to retain its critical dimensions were found to be replicated within 5%
phenotypic function under condition that mimics the accuracy from design values. Therefore, the SLA technique
native in vivo environment. 34,57 Such environment can has proven to be a quick and versatile solution to fabricate
be achieved through the development of 3D fibrous prototype devices with added functionalities and obviates
59
scaffold, incorporation of bioreactors and growth factors excessive secondary assembly and bonding processes.
42
that will lead to the development of functional tissue. The effect of media perfusion on the pore size and
56
Considering the requirement needed to provide a more porosity of the embedded 3D miniaturized scaffold was
physiologically relevant microenvironment for cell culture investigated. It was found that both pore size and porosity of
studies, a fibrous and porous scaffold was embedded onto a the embedded scaffold decrease minimally after perfusion.
3D-printed MPC device. The biological functional porous Such differences in pore size did not affect the subsequent
scaffold was used to recapitulate the structure aspect of the Huh7.5 cells viability studies as the confocal z-stack
ECM of a native tissue. imaging results show cellular infiltration of scaffold beyond
Volume 10 Issue 1 (2024) 152 https://doi.org/10.36922/ijb.0226