Page 215 - IJB-10-1
P. 215
International Journal of Bioprinting CS-laden microporous bio-ink for cartilage regeneration
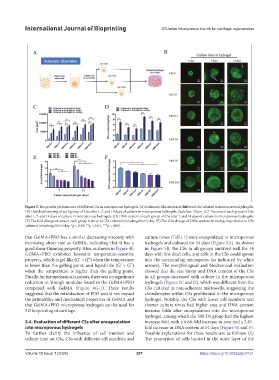
Figure 5. The growth performance of different CSs in microporous hydrogels. (A) Schematic illustration of different CSs cultured in microporous hydrogels.
(B) Live/dead staining of each group of CSs after 1, 7, and 14 days of culture in microporous hydrogels. Scale bar: 50 μm. (C) The area of each group of CSs
after 1, 7, and 14 days of culture in microporous hydrogels. (D) DNA content of each group of CSs after 1 and 14 days of culture in microporous hydrogels.
(E) The fold change of area in each group relative to CSs cultured in hydrogel for 0 day. (F) The fold change of DNA content in each group relative to CSs
cultured in hydrogel for 0 day. *p < 0.05; **p < 0.01; ***p < 0.001.
that GelMA+PEO has a similar decreasing viscosity with culture times (Table 1) were encapsulated in microporous
increasing shear rate as GelMA, indicating that it has a hydrogels and cultured for 14 days (Figure 5A). As shown
good shear-thinning property. Also, as shown in Figure 4F, in Figure 5B, the CSs in all groups survived well for 14
GelMA+PEO exhibited favorable temperature-sensitive days with few dead cells, and cells in the CSs could sprout
property, which is gel-like (G’ > G”) when the temperature into the surrounding micropores (as indicated by white
is lower than the gelling point, and liquid-like (G’ < G”) arrows). The morphological and biochemical evaluation
when the temperature is higher than the gelling point. showed that the size (area) and DNA content of the CSs
Finally, for the mechanical analysis, there was no significant in all groups increased with culture in the microporous
reduction in Young’s modulus found in the GelMA+PEO hydrogels (Figure 5C and D), which was different from the
compared with GelMA (Figure 4G–I). These results CSs cultured in non-adherent microwells, suggesting the
suggested that the introduction of PEO would not impact chondrocytes within CSs proliferated in the microporous
the printability and mechanical properties of GelMA and hydrogel. Notably, the CSs with lower cell numbers and
that GelMA+PEO microporous hydrogels can be used for shorter culture times had higher area and DNA content
3D bioprinting of cartilage. increase folds after encapsulation into the microporous
hydrogel, among which the 500 D1 group had the highest
3.4. Evaluation of different CSs after encapsulation increase fold, with a 6.64-fold increase in area and a 2.87-
into microporous hydrogels fold increase in DNA content at 14 days (Figure 5E and F).
To further clarify the influence of cell number and Possible explanations for these results are as follows: (1)
culture time on CSs, CSs with different cell numbers and The proportion of cells located in the outer layer of the
Volume 10 Issue 1 (2024) 207 https://doi.org/10.36922/ijb.0161