Page 216 - IJB-10-1
P. 216
International Journal of Bioprinting CS-laden microporous bio-ink for cartilage regeneration
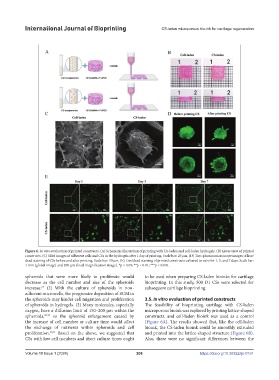
Figure 6. In vitro evaluation of printed constructs. (A) Schematic illustration of printing with CS-laden and cell-laden hydrogels. (B) Gross view of printed
constructs. (C) SEM images of adherent cells and CSs in the hydrogels after 1 day of printing. Scale bar: 25 μm. (D) Two-photon microscope images of live/
dead staining of CSs before and after printing. Scale bar: 50 μm. (E) Live/dead staining of printed constructs cultured in vitro for 1, 3, and 7 days. Scale bar:
1 mm (global image) and 200 μm (local magnification image). *p < 0.05; **p < 0.01; ***p < 0.001.
spheroids that were more likely to proliferate would to be used when preparing CS-laden bioinks for cartilage
decrease as the cell number and size of the spheroids bioprinting. In this study, 500 D1 CSs were selected for
increase. (2) With the culture of spheroids in non- subsequent cartilage bioprinting.
27
adherent microwells, the progressive deposition of ECM in
the spheroids may hinder cell migration and proliferation 3.5. In vitro evaluation of printed constructs
of spheroids in hydrogels. (3) Many molecules, especially The feasibility of bioprinting cartilage with CS-laden
oxygen, have a diffusion limit of 150–200 µm within the microporous bioink was explored by printing lattice-shaped
spheroids, 28,29 so the spheroid enlargement caused by constructs, and cell-laden bioink was used as a control
the increase of cell number or culture time would affect (Figure 6A). The results showed that, like the cell-laden
the exchange of nutrients within spheroids and cell bioink, the CS-laden bioink could be smoothly extruded
proliferation. 30,31 Based on the above, we suggested that and printed into the lattice-shaped structure (Figure 6B).
CSs with low cell numbers and short culture times ought Also, there were no significant differences between the
Volume 10 Issue 1 (2024) 208 https://doi.org/10.36922/ijb.0161