Page 420 - IJB-10-1
P. 420
International Journal of Bioprinting Osteogenic differentiation of hMSCs by PBF-LB
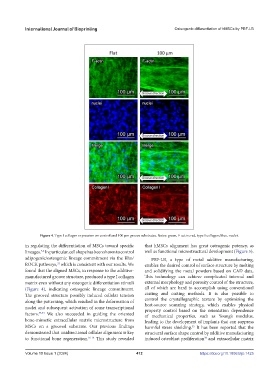
Figure 4. Type I collagen expression on control and 100 μm groove substrates. Notes: green, F-actin; red, type I collagen; blue, nuclei.
in regulating the differentiation of MSCs toward specific that hMSCs alignment has great osteogenic potency, as
5,6
lineages. In particular, cell shape has been shown to control well as functional microstructural development (Figure 5).
adipogenic/osteogenic lineage commitment via the Rho/ PBF-LB, a type of metal additive manufacturing,
ROCK pathways, which is consistent with our results. We enables the desired control of surface structure by melting
32
found that the aligned MSCs, in response to the additive- and solidifying the metal powders based on CAD data.
manufactured groove structure, produced a type I collagen This technology can achieve complicated internal and
matrix even without any osteogenic differentiation stimuli external morphology and porosity control of the structure,
(Figure 4), indicating osteogenic lineage commitment. all of which are hard to accomplish using conventional
The grooved structure possibly induced cellular tension casting and cutting methods. It is also possible to
along the patterning, which resulted in the deformation of control the crystallographic texture by optimizing the
nuclei and subsequent activation of some transcriptional heat-source scanning strategy, which enables physical
property control based on the orientation dependence
factors. 33,34 We also succeeded in guiding the oriented of mechanical properties, such as Young’s modulus,
bone-mimetic extracellular matrix microstructure from leading to the development of implants that can suppress
MSCs on a grooved substrate. Our previous findings harmful stress shielding. It has been reported that the
35
demonstrated that unidirectional cellular alignment is key structural surface shape control by additive manufacturing
to functional bone regeneration. 16-18 This study revealed induced osteoblast proliferation and extracellular matrix
36
Volume 10 Issue 1 (2024) 412 https://doi.org/10.18063/ijb.1425