Page 431 - IJB-10-1
P. 431
International Journal of Bioprinting Mechanically biomimicking 3D bone model
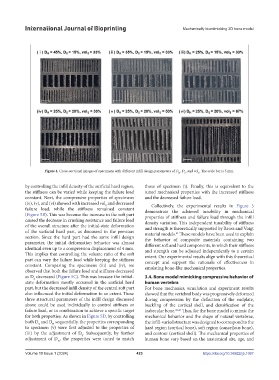
Figure 4. Cross-sectional images of specimens with different infill design parameters of D , D , and vol . The scale bar is 5 mm.
H S S
by controlling the infill density of the surficial hard region, those of specimen (i). Finally, this is equivalent to the
the stiffness can be varied while keeping the failure load tuned mechanical properties with the increased stiffness
constant. Next, the compressive properties of specimens and the decreased failure load.
(iv), (v), and (vi) showed with increased vol and decreased Collectively, the experimental results in Figure 5
S
failure load, while the stiffness remained constant demonstrate the achieved tunability in mechanical
(Figure 5B). This was because the increase in the soft part properties of stiffness and failure load through the infill
caused the decrease in crushing resistance and failure load density variation. This independent tunability of stiffness
of the overall structure after the initial-state deformation and strength is theoretically supported by Reuss and Voigt
of the surficial hard part, as discussed in the previous material models. These models have been used to explain
41
section. Since the hard part had the same infill design the behavior of composite materials containing two
parameter, the initial deformation behavior was almost different soft and hard components, in which their stiffness
identical even up to a compression displacement of 4 mm. and strength can be adjusted independently to a certain
This implies that controlling the volume ratio of the soft extent. Our experimental results align with this theoretical
part can vary the failure load while keeping the stiffness concept and support the rationale of effectiveness in
constant. Comparing the specimens (iii) and (iv), we emulating bone-like mechanical properties.
observed that both the failure load and stiffness decreased
as D decreased (Figure 5C). This was because the initial- 3.4. Bone model mimicking compressive behavior of
S
state deformation mostly occurred in the surficial hard human vertebra
part, but the decreased infill density of the central soft part For bone mechanics, simulation and experiment results
also influenced the initial deformation to an extent. These showed that the vertebral body was progressively deformed
three structural parameters of the infill design discussed during compression by the deflection of the endplate,
above could be used individually to control stiffness or buckling of the cortical shell, and densification of the
failure load, or in combination to achieve a specific target trabecular bone. 42,43 Thus, for the bone model to mimic the
for both properties. As shown in Figure 5D, by controlling mechanical behavior and the shape of natural vertebrae,
both D and D sequentially, the properties corresponding the infill-varied structure was designed to correspond to the
H
S
to specimen (v) were first adjusted to the properties of hard region (cortical bone), soft region (cancellous bone),
(iii) by the adjustment of D . Subsequently, by further and contour (cortical shell). The mechanical properties of
S
adjustment of D , the properties were tuned to match human bone vary based on the anatomical site, age, and
H
Volume 10 Issue 1 (2024) 423 https://doi.org/10.36922/ijb.1067