Page 82 - IJB-2-1
P. 82
Microstereolithography-fabricated microneedles for fluid sampling of histamine-contaminated tuna
croneedle sampling system procedure and the manufa- needle sampling system utilizes only 1 mL of solution.
cturer-described procedure. The positive results were In addition, the amount of time required to perform
initially detected using the microneedle procedure; the microneedle sampling system procedure was much
four independent test strips were used to assess that lower than the time required to perform the commer-
particular piece of tuna. Upon obtaining these unex- cially available histamine detection kit procedure. The
pected positive results, the histamine detection proce- commercially available histamine detection kit proce-
®
dure outlined by the commercial Reveal test kits was dure involves weighing 10 g of the sample, homoge-
used to assess whether or not the tuna contained his- nization of the sample, addition of water, mixing, di-
tamine. Four independent test strips were conducted lution, and use of the lateral flow test strip assay. On
on the tuna sample, which indicated that the tuna the other hand, the microneedle sampling system pro-
sample was spoiled and contained levels of histamine cedure involved application of the microneedle array
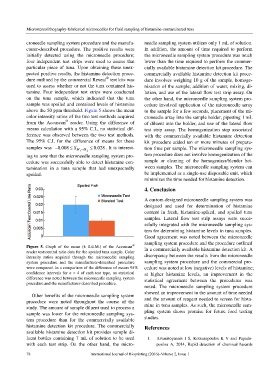
above the 50 ppm threshold. Figure 5 shows the mean to the sample for a few seconds, placement of the mi-
color intensity ratios of the two test methods acquired croneedle array into the sample holder, pipetting 1 mL
®
from the Accuscan reader. Using the difference of of diluent into the holder, and use of the lateral flow
means calculation with a 95% C.I., no statistical dif- test strip assay. The homogenization step associated
ference was observed between the two test methods. with the commercially available histamine detection
The 95% C.I. for the difference of means for these kit procedure added ten or more minutes of prepara-
samples was − 0.008 ≤ S M 1 M− 2 ≤ 0.028. It is interest- tion time per sample. The microneedle sampling sys-
ing to note that the microneedle sampling system pro- tem procedure does not involve homogenization of the
cedure was successfully able to detect histamine con- sample or cleaning of the homogenizer/blender bet-
tamination in a tuna sample that had unexpectedly ween samples. The microneedle sampling system can
spoiled. be implemented as a single-use disposable unit, which
minimizes the time needed for histamine detection.
4. Conclusion
A custom-designed microneedle sampling system was
designed and used for determination of histamine
content in fresh, histamine-spiked, and spoiled tuna
samples. Lateral flow test strip assays were succe-
ssfully integrated with the microneedle sampling sys-
tem for determining histamine levels in tuna samples.
Good agreement was noted between the microneedle
sampling system procedure and the procedure outlined
®
Figure 5. Graph of the mean (± S.E.M.) of the Accuscan
reader test:control ratio data for the spoiled tuna sample. Color in a commercially available histamine detection kit. A
intensity ratios acquired through the microneedle sampling discrepancy between the results from the microneedle
system procedure and the manufacturer-described procedure sampling system procedure and the commercial pro-
were compared. In a comparison of the difference of means 95% cedure was noted at low (negative) levels of histamine;
confidence intervals for n = 4 of each test type, no statistical at higher histamine levels, an improvement in the
difference was noted between the microneedle sampling system statistical agreement between the procedures was
procedure and the manufacturer-described procedure.
noted. The microneedle sampling system procedure
Other benefits of the microneedle sampling system showed an improvement in the amount of time needed
procedure were noted throughout the course of the and the amount of reagent needed to screen for hista-
study. The amount of sample diluent used to process a mine in tuna samples. As such, the microneedle sam-
sample was lower for the microneedle sampling sys- pling system shows promise for future food testing
tem procedure than for the commercially available studies.
histamine detection kit procedure. The commercially References
available histamine detection kit provides sample di-
luent bottles containing 7 mL of solution to be used 1. Arvanitoyannis I S, Kotsanopoulos K V and Papado-
with each test strip. On the other hand, the micro- poulou A, 2014, Rapid detection of chemical hazards
78 International Journal of Bioprinting (2016)–Volume 2, Issue 1