Page 41 - IJB-3-1
P. 41
Hyeong-jin Lee, Young Won Koo, Miji Yeo, et al.
fore, collagen bioink has been investigated to improve the 3D cell printing process. To date, the development
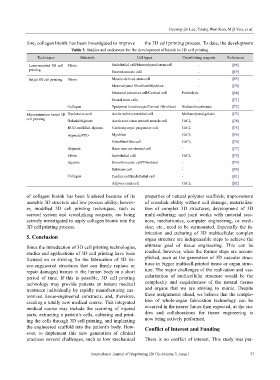
Table 3. Studies and endeavors for the development of bioink in 3D cell printing
Techniques Materials Cell types Crosslinking reagents References
Laser-assisted 3D cell Fibrin Endothelial cell/Mesenchymal stem cell - [68]
printing Smooth muscle cell - [69]
Inkjet 3D cell printing Fibrin Muscle-derived stem cell - [65]
Mesenchymal fibroblast/Myoblast - [70]
Neuronal precursor cell/Cortical cell Proteolytic [64]
Neural stem cells - [71]
Collagen Epidermal keratinocyte/Dermal fibro blast Sodium bicarbonate [72]
Microextrusion based 3D Hyaluronic acid Aortic valve interstitial cell Methacrylated gelatin [73]
cell printing Gelatin/Alginate Aortic root sinus smooth muscle cell CaCl 2 [74]
RGD-modified alginate Cardiomyocyte progenitor cell CaCl 2 [75]
Alginate/PEO Myoblast CaCl 2 [56]
Osteoblast-like cell CaCl 2 [76]
Alginate Bone marrow stromal cell - [77]
Fibrin Endothelial cell CaCl 2 [78]
Agarose Smooth muscle cell/Fibroblast - [79]
Schwann cell - [80]
Collagen Cardiac cell/Endothelial cell - [81]
Adipose stem cell CaCl 2 [82]
of collagen bioink has been hindered because of its properties of natural polymer scaffolds; improvement
unstable 3D structure and low process ability; howev- of crosslink ability without cell damage; materializa-
er, modified 3D cell printing techniques, such as tion of complex 3D structures; development of 3D
aerosol system and crosslinking reagents, are being multi-culturing; and joint works with material scie-
actively investigated to apply collagen bioink into the nces, mechatronics, computer engineering, or medi-
3D cell printing process. cine; etc., need to be surmounted. Especially the fa-
brication and culturing of 3D multicellular complex
5. Conclusion organ structure are indispensable steps to achieve the
Since the introduction of 3D cell printing technologies, ultimate goal of tissue engineering. This can be
studies and applications of 3D cell printing have been reached, however, when the former steps are accom-
focused on or striving for the fabrication of 3D tis- plished, such as the generation of 3D vascular struc-
sue-engineered structures that can firmly replace or tures in bigger multicell-printed tissue or organ struc-
repair damaged tissues in the human body in a short ture. The major challenges of the realization and vas-
period of time. If this is possible, 3D cell printing cularization of multicellular structure would be the
technology may provide patients an instant medical complexity and exquisiteness of the natural tissues
treatment individually by rapidly manufacturing cus- and organs that we are striving to mimic. Despite
tomized tissue-engineered constructs, and, therefore, these assignments ahead, we believe that the comple-
creating a totally new medical course. This integrated tion of whole-organ fabrication technology can be
medical course may include the scanning of injured occurred in the nearer future than expected, as the stu-
parts, extracting a patient’s cells, culturing and print- dies and collaborations for tissue engineering is
ing the cells through 3D cell printing, and implanting now being actively performed.
the engineered scaffold into the patient’s body. How- Conflict of Interest and Funding
ever, to implement this new generation of clinical
practices several challenges, such as low mechanical There is no conflict of interest. This study was par-
International Journal of Bioprinting (2017)–Volume 3, Issue 1 37