Page 491 - IJB-10-3
P. 491
International Journal of Bioprinting Stretchable scaffold for modeling fibrosis
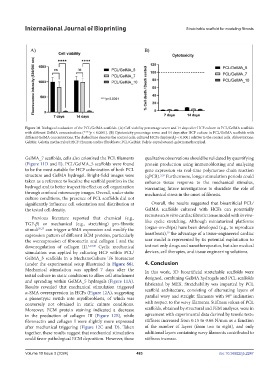
Figure 10. Biological evaluation of the PCL/GelMA scaffolds. (A) Cell viability percentage seven and 14 days after HCF culture in PCL/GelMA scaffolds
with different GelMA concentrations (****p < 0.0001). (B) Cytotoxicity percentage seven and 14 days after HCF culture in PCL/GelMA scaffolds with
different GelMA concentrations. The dashed line denotes the control cells; cultured HCFs displayed p < 0.0001 relative to the control cells. Abbreviations:
GelMA: Gelatin methacryloyl; HCF: Human cardiac fibroblasts; PCL/GelMA: Poly(ε-caprolactone)-gelatin methacryloyl.
GelMA_7 scaffolds, cells also colonized the PCL filaments qualitative observations should be validated by quantifying
(Figure 11D and E). PCL/GelMA_5 scaffolds were found protein production using immunoblotting and analyzing
to be the most suitable for HCF colonization of both PCL gene expression via real-time polymerase chain reaction
structure and GelMA hydrogel. Bright-field images were (qPCR). Furthermore, longer stimulation periods could
5,29
taken as a reference to localize the scaffold position in the enhance tissue response to the mechanical stimulus,
hydrogel and to better inspect its effect on cell organization warranting future investigations to elucidate the role of
through confocal microscopy images. Overall, under static mechanical stress in the onset of fibrosis.
culture conditions, the presence of PCL scaffolds did not
significantly influence cell orientation and distribution at Overall, the results suggested that bioartificial PCL/
the tested cell density. GelMA scaffolds cultured with HCFs can potentially
recreate an in vitro cardiac fibrotic tissue model with in vivo-
Previous literature reported that chemical (e.g.,
TGF-β) or mechanical (e.g., stretching) pro-fibrotic like cyclic stretching. Although miniaturized platforms
stimuli 29,37 can trigger α-SMA expression and modify the (organ-on-chips) have been developed (e.g., to reproduce
29
expression pattern of different ECM proteins, particularly heartbeats), the advantage of a tissue-engineered cardiac
the overexpression of fibronectin and collagen I and the scar model is represented by its potential exploitation to
downregulation of collagen III. 5,37,48 Cyclic mechanical test not only drugs and nanotherapeutics, but also medical
stimulation was applied by culturing HCF within PCL/ devices, cell therapies, and tissue engineering solutions.
GelMA_5 scaffolds in a MechanoCulture T6 bioreactor
(under the experimental setup illustrated in Figure S6). 4. Conclusion
Mechanical stimulation was applied 7 days after the In this work, 3D bioartificial stretchable scaffolds were
initial culture in static conditions to allow cell attachment designed, combining GelMA hydrogels and PCL scaffolds
and spreading within GelMA_5 hydrogels (Figure 11A). fabricated by MEX. Stretchability was imparted by PCL
Results revealed that mechanical stimulation triggered
α-SMA overexpression in HCFs (Figure 12A), suggesting scaffold architecture, consisting of alternating layers of
a phenotypic switch into myofibroblasts, of which was parallel wavy and straight filaments with 90° inclination
conversely not obtained in static culture conditions. with respect to the wavy filaments. Stiffness values of PCL
Moreover, ECM protein staining indicated a decrease scaffolds, obtained by structural and FEM analyses, were in
in the production of collagen III (Figure 12B), while agreement with experimental data derived by tensile tests:
fibronectin and collagen I were slightly more expressed stiffness increased from 0.15 to 0.66 N/mm as a function
after mechanical triggering (Figure 12C and D). Taken of the number of layers (from two to eight), and only
together, these results suggest that mechanical stimulation additional layers containing wavy filaments contributed to
could favor pathological ECM deposition. However, these stiffness increase.
Volume 10 Issue 3 (2024) 483 doi: 10.36922/ijb.2247