Page 71 - IJB-4-1
P. 71
An nMgO containing scaffold: Antibacterial activity, degradation properties and cell responses
(A) (B) (C)
(D) (E) (F)
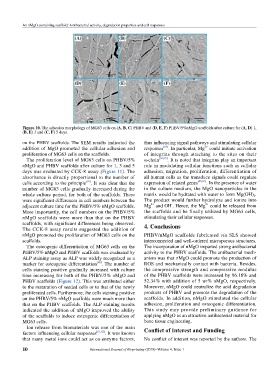
Figure 10. The adhesion morphology of MG63 cells on (A, B, C) PHBV and (D, E, F) PHBV/5%nMgO scaffolds after culture for (A, D) 1,
(B, E) 3 and (C, F) 5 days.
on the PHBV scaffolds. The SEM results indicated the thus influencing signal pathways and stimulating cellular
[63]
addition of MgO promoted the cellular adhesion and responses . In particular, Mg could initiate activation
2+
proliferation of MG63 cells on the scaffolds. of integrins through attaching to the sites on their
The proliferation level of MG63 cells on PHBV/5% α-chain [64,65] . It is noted that integrins play an important
nMgO and PHBV scaffolds after culture for 1, 3 and 5 role in modulating cellular functions such as cellular
days was evaluated by CCK-8 assay (Figure 11). The adhesion, migration, proliferation, differentiation of
absorbance is directly proportional to the number of all human cells as the transduce signals could regulate
[59]
cells according to the principle . It was clear that the expression of related genes [66,67] . In the presence of water
number of MG63 cells gradually increased during the in the culture medium, the MgO nanoparticles in the
whole culture period, for both of the scaffolds. There matrix would be hydrated with water to form Mg(OH) .
2
were significant differences in cell numbers between the The product would further hydrolyze and ionize into
2+
-
2+
adjacent culture time for the PHBV/5% nMgO scaffolds. Mg and OH . Hence, the Mg could be released from
More importantly, the cell numbers on the PHBV/5% the scaffolds and be finally utilized by MG63 cells,
nMgO scaffolds were more than that on the PHBV stimulating their cellular responses.
scaffolds, with significant differences being observed. 4. Conclusions
The CCK-8 assay results suggested the addition of
nMgO promoted the proliferation of MG63 cells on the PHBV/nMgO scaffolds fabricated via SLS showed
scaffolds. interconnected and well-ordered microporous structures.
The osteogenic differentiation of MG63 cells on the The incorporation of nMgO imparted strong antibacterial
PHBV/5% nMgO and PHBV scaffolds was evaluated by activity to the PHBV scaffolds. The antibacterial mech-
ALP staining assay as ALP was widely recognized as a a nism was that nMgO could promote the production of
[60]
marker for osteogenic differentiation . The number of ROS and mechanically contact with bacteria. Besides,
cells staining positive gradually increased with culture the compressive strength and compressive modulus
time increasing for both of the PHBV/5% nMgO and of the PHBV scaffolds were increased by 96.18% and
PHBV scaffolds (Figure 12). This was attributed either 52.34% with addition of 5 wt% nMgO, respectively.
to the maturation of seeded cells or to that of the newly Moreover, nMgO could neutralize the acid degradation
proliferated cells. Furthermore, the cells staining positive products of PHBV and promote the degradation of the
on the PHBV/5% nMgO scaffolds were much more than scaffolds. In addition, nMgO stimulated the cellular
that on the PHBV scaffolds. The ALP staining results adhesion, proliferation and osteogenic differentiation.
indicated the addition of nMgO improved the ability This study may provide preliminary guidance for
of the scaffolds to induce osteogenic differentiation of applying nMgO as an attractive antibacterial material for
MG63 cells. bone tissue engineering.
Ion release from biomaterials was one of the main Conflict of Interest and Funding
factors influencing cellular responses [61,62] . It was known
that many metal ions could act as co-enzyme factors, No conflict of interest was reported by the authors. The
10 International Journal of Bioprinting (2018)–Volume 4, Issue 1