Page 13 - IJB-4-2
P. 13
Lee J M, et al.
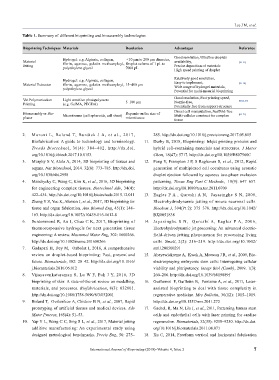
Table 1. Summary of different bioprinting and bioassembly technologies
Bioprinting Techniques Materials Resolution Advantages Reference
Good resolution, Ultrafine droplets
Material Hydrogel: e.g. Alginate, collagen, <10 µm to 200 µm diameter, availability, [18–21]
Jetting fibrin, agarose, gelatin methacryloyl, Droplet volume of 1 pL to Precise deposition of materials
polyethylene glycol 7000 pL High speed printing of droplet
Hydrogel: e.g. Alginate, collagen, Relatively good resolution,
Material Extrusion fibrin, agarose, gelatin methacryloyl, 15–400 µm Easy to implement, [29–38]
polyethylene glycol Wide range of hydrogel materials,
Potential for multi-material bioprinting
Good resolution, Fast printing speed,
Vat Polymerization Light sensitive photopolymers 5–100 µm Nozzle-free, [60,61,67]
Printing (e.g. GelMA, PEGDA) Potentially free from support-structure
Bioassembly or Bio- Depends on the size of Direct cell manipulation, Scaffold-free [69–71]
placer Microtissues (cell spheroids, cell sheet) microtissues Multi-cellular construct for complex
tissue
2. Moroni L, Boland T, Burdick J A, et al., 2017, 285. http://dx.doi.org/10.1016/j.precisioneng.2017.05.015
Biofabrication: A guide to technology and terminology. 11. Derby B, 2018, Bioprinting: Inkjet printing proteins and
Trends Biotechnol, 36(4): 384–402. http://dx.doi. hybrid cell-containing materials and structures. J Mater
org/10.1016/j.tibtech.2017.10.0153. Chem, 18(47): 5717. http://dx.doi.org/10.1039/B807560C
3. Murphy S V, Atala A, 2014, 3D bioprinting of tissues and 12. Fang Y, Frampton J P, S Raghavan S, et al., 2012, Rapid
organs. Nat Biotechnol, 2014. 32(8): 773–785. http://dx.doi. generation of multiplexed cell cocultures using acoustic
org/10.1038/nbt.2958 droplet ejection followed by aqueous two-phase exclusion
4. Mandrycky C, Wang C, Kim K, et al., 2016, 3D bioprinting patterning. Tissue Eng Part C Methods, 18(9): 647–657.
for engineering complex tissues. Biotechnol Adv, 34(4): http://dx.doi.org/10.1089/ten.tec.2011.0709
422–434. http://dx.doi.org/10.1016/j.biotechadv.2015.12.011 13. Eagles P A , Qureshi A N, Jayasinghe S N, 2006,
5. Zhang Y S, Yue K, Aleman J, et al., 2017, 3D bioprinting for Electrohydrodynamic jetting of mouse neuronal cells.
tissue and organ fabrication. Ann Biomed Eng, 45(1): 148– Biochem J, 394(Pt 2): 375–378. http://dx.doi.org/10.1042/
163. http://dx.doi.org/10.1007/s10439-016-1612-8 BJ20051838
6. Suntornnond R, An J, Chua C K, 2017, Bioprinting of 14. Jayasinghe S N, Qureshi A, Eagles P A, 2006,
thermoresponsive hydrogels for next generation tissue Electrohydrodynamic jet processing: An advanced electric-
engineering: A review. Macromol Mater Eng, 302: 1600266. field-driven jetting phenomenon for processing living
http://dx.doi.org/10.1002/mame.201600266 cells. Small, 2(2): 216–219. http://dx.doi.org/10.1002/
7. Gudapati H, Dey M, Ozbolat I, 2016, A comprehensive smll.200500291
review on droplet-based bioprinting: Past, present and 15. Abeyewickreme A, Kwok A, Mcewan J R, et al., 2009, Bio-
future. Biomaterials, 102: 20–42. http://dx.doi.org/10.1016/ electrospraying embryonic stem cells: Interrogating cellular
j.biomaterials.2016.06.012 viability and pluripotency. Integr Biol (Camb), 2009. 1(3):
8. Vijayavenkataraman S, Lu W F, Fuh J Y, 2016, 3D 260–266. http://dx.doi.org/10.1039/b819889f
bioprinting of skin: A state-of-the-art review on modelling, 16. Guillemot F, Guillotin B, Fontaine A, et al., 2011, Laser-
materials, and processes. Biofabrication, 8(3): 032001. assisted bioprinting to deal with tissue complexity in
http://dx.doi.org/10.1088/1758-5090/8/3/032001 regenerative medicine. Mrs Bulletin, 36(12): 1015–1019.
9. Boland T, Ovslanikov A, Chickov B N, et al., 2007, Rapid http://dx.doi.org/10.1557/mrs.2011.272
prototyping of artificial tissues and medical devices. Adv 17. Gaebel, R, Ma N, Liu J, et al., 2011, Patterning human stem
Mater Process, 165(4): 51–53. cells and endothelial cells with laser printing for cardiac
10. Yap Y L, Wang C C, Sing S L, et al., 2017, Material jetting regeneration. Biomaterials, 32(35): 9218–9230. http://dx.doi.
additive manufacturing: An experimental study using org/10.1016/j.biomaterials.2011.08.071
designed metrological benchmarks. Precis Eng, 50: 275– 18. Xu C, 2014, Freeform vertical and horizontal fabrication
International Journal of Bioprinting (2018)–Volume 4, Issue 2 7