Page 73 - IJB-4-2
P. 73
Novel ultrashort self-assembling peptide bioinks for 3D culture of muscle myoblast cells
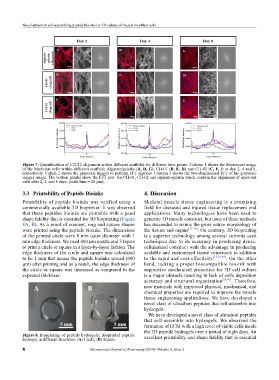
Figure 7. Quantification of C2C12 alignment within different scaffolds for different time points. Column 1 shows the fluorescent image
of the Myoblast cells within different scaffold; Alginate/gelatin (A, D, G), CH-01 (B, E, H) and CH-02 (C, F, I) at day 2, 4 and 8,
respectively. Colum 2 shows the grayscale images to perform FFT analysis. Column 3 shows the two-dimensional FFT of the grayscale
images image. The bottom graphs show the FFT plot for CH-01, CH-02 and alginate-gelatin which confirm the alignment of myoblast
cells after 2, 4, and 8 days, (scale bars = 20 µm).
3.3 Printability of Peptide Bioinks 4. Disscusion
Printability of peptide bioinks was verified using a Skeletal muscle tissue engineering is a promising
commercially available 3D bioprinter. It was observed field for diseased and injured tissue replacement and
that these peptides bioinks are printable with a good applications. Many technologies have been used to
shape fidelity that is essential for 3D bioprinting (Figure generate 3D muscle construct, but none of these methods
8A, B). As a proof of concept, ring and square shapes has succeeded to mimic the gross native morphology of
were printed using the peptide bioinks. The dimensions the tissues and organs [37–39] . On contrary, 3D bioprinting
of the printed circle were 8 mm outer diameter with 1 is a superior technology among several currents used
mm edge thickness. We used 400 µm nozzle and 3 layers techniques due to its accuracy in producing dense,
to print a circle or square in a layer-by-layer fashion. The cellularized construct with the advantage in producing
edge thickness of the circle and square was calculated scalable and customized tissue constructs in addition
to be 1 mm that means the peptide bioinks spread (600 to the rapid and cost-effectivity [15,26,40] . On the other
µm) after printing and as a result, the edge thickness of hand, lacking a proper biocompatible bio-ink with
the circle or square was increased as compared to the supportive mechanical properties for 3D cell culture
expected thickness. is a major obstacle resulting in lack of cells deposition
accuracy and structural organization [26,41] . Therefore,
new materials with improved physical, mechanical, and
chemical properties are required to improve the muscle
tissue engineering applications. We have developed a
novel class of ultrashort peptides that self-assemble into
hydrogels.
We have developed a novel class of ultrashort peptides
that self-assemble into hydrogels. We observed the
formation of ECM with a high level of viable cells inside
the 3D peptide hydrogels over a period of eight days. An
Figure 8. Bioprinting of peptide hydrogels; Bioprinted peptide
hydrogel in different structures: (A) Circle; (B) Square. excellent printability and shape fidelity that is essential
8 International Journal of Bioprinting (2018)–Volume 4, Issue 2