Page 315 - IJB-10-4
P. 315
International Journal of Bioprinting PCL/Fe3O4@ZIF-8 for infected bone repair
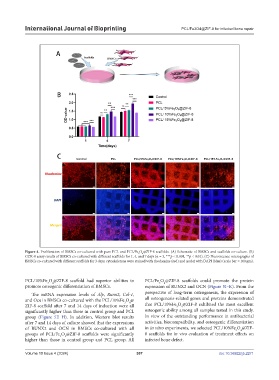
Figure 4. Proliferation of BMSCs co-cultured with pure PCL and PCL/Fe O @ZIF-8 scaffolds. (A) Schematic of BMSCs and scaffolds co-culture. (B)
4
3
CCK-8 assay results of BMSCs co-cultured with different scaffolds for 1, 4, and 7 days (n = 3, ***p < 0.001, **p < 0.01). (C) Fluorescence micrographs of
BMSCs co-cultured with different scaffolds for 3 days; cytoskeletons were stained with rhodamine (red) and nuclei with DAPI (blue) (scale bar = 100 µm).
PCL/10%Fe O @ZIF-8 scaffold had superior abilities to PCL/Fe O @ZIF-8 scaffolds could promote the protein
4
4
3
3
promote osteogenic differentiation of BMSCs. expression of RUNX2 and OCN (Figure 5I–K). From the
The mRNA expression levels of Alp, Runx2, Col-1, perspective of long-term osteogenesis, the expression of
and Ocn in BMSCs co-cultured with the PCL/10%Fe O @ all osteogenesis-related genes and proteins demonstrated
3
4
ZIF-8 scaffold after 7 and 14 days of induction were all that PCL/10%Fe O @ZIF-8 exhibited the most excellent
3
4
significantly higher than those in control group and PCL osteogenic ability among all samples tested in this study.
group (Figure 5E–H). In addition, Western blot results In view of the outstanding performance in antibacterial
after 7 and 14 days of culture showed that the expressions activities, biocompatibility, and osteogenic differentiation
of RUNX2 and OCN in BMSCs co-cultured with all in in vitro experiments, we selected PCL/10%Fe O @ZIF-
3
4
groups of PCL/Fe O @ZIF-8 scaffolds were significantly 8 scaffolds for in vivo evaluation of treatment effects on
4
3
higher than those in control group and PCL group. All infected bone defect.
Volume 10 Issue 4 (2024) 307 doi: 10.36922/ijb.2271