Page 110 - IJB-5-2
P. 110
A methodology to develop a vascular geometry for in vitro cell culture using additive manufacturing
5. Observation and biologist Feedback monolayer with confluence was observed (Figure 10).
Human umbilical vein endothelial cells (HUVECs) A weak flow rate perfusion (Figure 11, shear stress 1
were seeded in a tube fabricated by two-half mold dyn/cm²) was then applicated on cells for 3H following
technique. After 2 h of static culture, a homogenous by immunostaining to visualize specific markers of
endothelial cells (PECAM-1, Platelet endothelial cell
adhesion molecule 1) and their nuclear (DAPI). The
results demonstrated that HUVECs monolayer was
still confluence (Figure 12).
As flow experiments in artificial carotid will be
performed at high shear stress, HUVECs monolayer
was challenged with flow culture at high flow rate
perfusion (shear stress 6.5 dyn/cm²). However, the assay
was stopped after 12 min because of medium leaking at
bonding site on the two-half tube. Moreover, on phase-
contrast microscopy, cells detachments were found in
many areas (Figure 13).
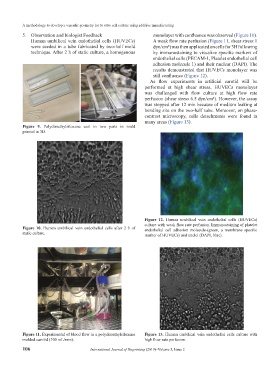
Figure 9. Polydimethylsiloxane cast in two parts in mold
printed in 3D.
Figure 12. Human umbilical vein endothelial cells (HUVECs)
culture with weak flow rate perfusion. Immunostaining of platelet
Figure 10. Human umbilical vein endothelial cells after 2 h of endothelial cell adhesion molecule-(green, a membrane specific
static culture. marker of HUVECs) and nuclei (DAPI, blue).
Figure 11. Experimental of blood flow in a polydimethylsiloxane Figure 13. Human umbilical vein endothelial cells culture with
molded carotid (100 mL/min). high flow rate perfusion.
106 International Journal of Bioprinting (2019)–Volume 5, Issue 2