Page 96 - IJB-5-2
P. 96
In vitro model of the glial scar
2.3 Macroscopic and Microcosmic 3. Results
Determination Collagen Gels
The collagen gel blocks of 10 × 10 × 2 mm were 3.1 Morphology and Proliferation of Astrocytes
3
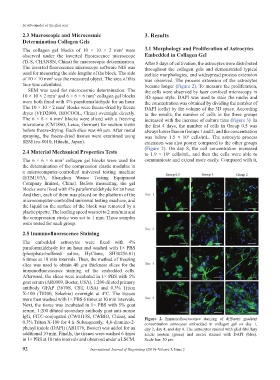
observed under the inverted fluorescence microscope Embedded in Collagen Gel
(Ti-S, CHANSN, China) for macroscopic determination. After 8 days of cultivation, the astrocytes were distributed
The inverted fluorescence microscope software NIS was throughout the collagen gels and demonstrated typical
used for measuring the side lengths of the block. The side stellate morphologies, and widespread process extension
of 10 × 10 mm was the measured object. The area of this was observed. The process extension of the astrocytes
2
face was calculated. became longer (Figure 2). To measure the proliferation,
SEM was used for microcosmic determination. The the cells were observed by laser confocal microscopy in
10 × 10 × 2 mm and 6 × 6 × 6 mm collagen gel blocks 3D space style. DAPI was used to stain the nuclei and
3
3
were both fixed with 4% paraformaldehyde for an hour. the concentration was obtained by dividing the number of
The 10 × 10 × 2 mm blocks were freeze-dried by freeze DAPI (cells) by the volume of the 3D space. According
3
dryer (VFD2000, BIOCOOL, China) overnight directly. to the results, the number of cells in the three groups
The 6 × 6 × 6 mm blocks were sliced with a freezing increased with the increase of culture time (Figure 3). In
3
microtome (CM1860, Leica, German) for section views the first 4 days, the number of cells in Group 0.5 was
before freeze-drying. Each slice was 40 μm. After metal always lower than in Groups 1 and 2, and the concentration
spraying, the freeze-dried tissues were examined using was below 1.5 × 10 cells/mL. The astrocyte process
6
SEM (su-8010, Hitachi, Japan). extension was also poorer compared to the other groups
(Figure 2). On day 8, the cell concentration increased
2.4 Material Mechanical Properties Tests to 1.9 × 10 cells/mL, and then the cells were able to
6
The 6 × 6 × 6 mm collagen gel blocks were used for communicate and extend more easily. Compared with it,
3
the determination of the compression elastic modulus in
a microcomputer-controlled universal testing machine
(ETM103A, Shenzhen Wance Testing Equipment
Company limited, China). Before measuring, the gel
blocks were fixed with 4% paraformaldehyde for an hour.
And then, each of them was placed on the platform of the
microcomputer-controlled universal testing machine, and
the liquid on the surface of the block was removed by a
plastic pipette. The loading speed was set to 2 mm/min and
the compression stroke was set to 1 mm. Three samples
were tested for each group.
2.5 Immunofluorescence Staining
The embedded astrocytes were fixed with 4%
paraformaldehyde for an hour and washed with 1× PBS
(phosphate-buffered saline, HyClone, SH30256.01)
6 times at 10 min intervals. Then, the method of freezing
slice was used to obtain 40 µm thickness slices for the
immunofluorescence staining of the embedded cells.
Afterward, the slices were incubated in 1× PBS with 5%
goat serum (AR0009, Boster, USA), 1:200 diluted primary
antibody GFAP (3670S, CST, USA) and 0.3% Triton
X-100 (T8200, Solarbio) overnight at 4°C. The tissues
were then washed with 1× PBS 6 times at 10 min intervals.
Next, the tissue was incubated in 1× PBS with 5% goat
serum, 1:200 diluted secondary antibody goat anti-mouse
IgG, FITC-conjugated (CW0113S, CWBIO, China), and Figure 2. Immunofluorescence staining of different gradient
0.3% Triton X-100 for 4 h. Subsequently, 4,6-diamino-2- concentration astrocytes embedded in collagen gel on day 1,
phenyl indole (DAPI) (AR1176, Boster) was added for an day 2, day 4, and day 8. The astrocytes stained with glial fibrillary
additional 10 min. Finally, the tissues were washed 6 times acidic protein (green) and nuclei stained with DAPI (blue).
in 1× PBS at 10 min intervals and observed under a LSCM. Scale bar: 50 μm.
92 International Journal of Bioprinting (2019)–Volume 5, Issue 2