Page 110 - IJB-6-1
P. 110
Matrix-assisted pulsed laser evaporation-deposited rapamycin thin films
such as (a) blinking, (b) low corneal membrane rapamycin or other pharmacologic agent-
permeability, (c) nasolacrimal drainage, (d) non- containing coatings (e.g., dip-coating, spin coating,
productive absorption through the conjunctiva, and Langmuir–Blodgett dip-coating) such as the
and (e) tearing . Due to the aforementioned facile control of film thickness, roughness and
[4]
issues, the use of eye drop solutions is associated uniformity, the deposition of thin films in a single
with a tear film residence time of 1–3 min and “step” , and the maintenance of pharmacologic
[12]
low bioavailability (1–3%) . This limitation can agent integrity. For example, poly(D, L) lactic
[4]
be addressed with frequent dosing; however, acid/dexamethasone bilayer coatings deposited
frequent dosing is associated with low patient using MAPLE significantly reduced nitric oxide
compliance and a high incidence of side effects . production in microglial cell cultures demonstrating
[9]
Alternatively, rapamycin can be loaded onto that the biological function of dexamethasone is
contact lenses to improve drug bioavailability. retained after the MAPLE process . In addition,
[13]
The most straightforward mechanism to load a MAPLE was previously utilized for the coverage
pharmacologic agent into contact lenses is by of non-planar substrates [14,15] .
soaking a preformed contact lens in a solution The MAPLE process involves laser evaporation
containing the pharmacologic agent before of a frozen solution containing the pharmacologic
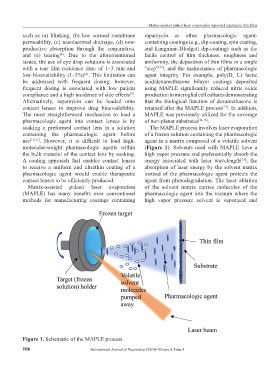
use [10,11] . However, it is difficult to load high- agent in a matrix composed of a volatile solvent
molecular-weight pharmacologic agents within (Figure 1). Solvents used with MAPLE have a
the bulk material of the contact lens by soaking. high vapor pressure and preferentially absorb the
A coating approach that enables contact lenses energy associated with laser wavelength ; the
[13]
to receive a uniform and ultrathin coating of a absorption of laser energy by the solvent matrix
pharmacologic agent would enable therapeutic instead of the pharmacologic agent protects the
contact lenses to be efficiently produced. agent from photodegradation. The laser ablation
Matrix-assisted pulsed laser evaporation of the solvent matrix carries molecules of the
(MAPLE) has many benefits over conventional pharmacologic agent into the vacuum where the
methods for manufacturing coatings containing high vapor pressure solvent is vaporized and
Figure 1. Schematic of the MAPLE process.
106 International Journal of Bioprinting (2020)–Volume 6, Issue 1