Page 340 - IJB-10-6
P. 340
International Journal of Bioprinting 3D-printed PPDO/GO stents for CHD treatment.
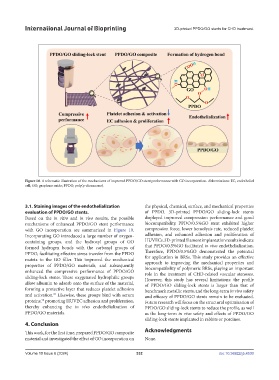
Figure 10. A schematic illustration of the mechanisms of improved PPDO/GO stent performance with GO incorporation. Abbreviations: EC, endothelial
cell; GO, graphene oxide; PPDO, poly(p-dioxanone).
3.1. Staining images of the endothelialization the physical, chemical, surface, and mechanical properties
evaluation of PPDO/GO stents. of PPDO. 3D-printed PPDO/GO sliding-lock stents
Based on the in vitro and in vivo results, the possible displayed improved compression performance and good
mechanisms of enhanced PPDO/GO stent performance biocompatibility. PPDO/0.5%GO stent exhibited higher
with GO incorporation are summarized in Figure 10. compression force, lower hemolysis rate, reduced platelet
Incorporating GO introduced a large number of oxygen- adhesion, and enhanced adhesion and proliferation of
containing groups, and the hydroxyl groups of GO HUVECs. 3D-printed filament implantation results indicate
formed hydrogen bonds with the carbonyl groups of that PPDO/0.5%GO facilitated in vivo endothelialization.
PPDO, facilitating effective stress transfer from the PPDO Therefore, PPDO/0.5%GO demonstrated the potential
matrix to the GO filler. This improved the mechanical for application in BRSs. This study provides an effective
properties of PPDO/GO materials, and subsequently approach to improving the mechanical properties and
enhanced the compressive performance of PPDO/GO biocompatibility of polymeric BRSs, playing an important
role in the treatment of CHD-related vascular stenoses.
sliding-lock stents. These oxygenated hydrophilic groups However, this study has several limitations: the profile
allow albumin to adsorb onto the surface of the material, of PPDO/GO sliding-lock stents is larger than that of
forming a protective layer that reduces platelet adhesion benchmark metallic stents, and the long-term in vivo safety
and activation. Likewise, these groups bind with serum and efficacy of PPDO/GO stents remain to be evaluated.
95
proteins, promoting HUVEC adhesion and proliferation, Future research will focus on the structural optimization of
98
thereby enhancing the in vivo endothelialization of PPDO/GO sliding-lock stents to reduce the profile, as well
PPDO/GO materials. as the long-term in vivo safety and effects of PPDO/GO
sliding-lock stents implanted in rabbits or porcines.
4. Conclusion
Acknowledgments
This work, for the first time, prepared PPDO/GO composite
material and investigated the effect of GO incorporation on None.
Volume 10 Issue 6 (2024) 332 doi: 10.36922/ijb.4530