Page 48 - IJB-7-3
P. 48
3D Bioprinting Photo-crosslinkable Hydrogels for Bone and Cartilage Repair
based bioprinting strategy (Figure 2B) . First, they used regeneration of cartilage is limited by its low number
[6]
[71]
bioprinting technology to imitate the overall structure of of cells . The difficulties of fabricating artificial
native bone tissue (Figure 2 Bi). Then, the GelMA hydrogel cartilaginous tissue can be solved by bioprinting because
cylinders were individually printed (Figure 2Bii). After this printing technique allows for encapsulation of
piling up these cylinders, pyramidal constructs were implanting cells. Meanwhile, the hydrogel has a striking
formed (Figure 2Biii). They also encapsulate angiogenic similarity to the ECM of natural cartilage . Thus, 3D
[72]
cells, and osteogenic cells and silicate nanoplatelets into bioprinted hydrogels become a potential alternative
the GelMA hydrogel simultaneously to promote bone therapy for cartilage repair. Duchi et al. developed a
regeneration ability. Through optimization of bioprinting hand-held extrusion-based bioprinter for 3D bioprinting
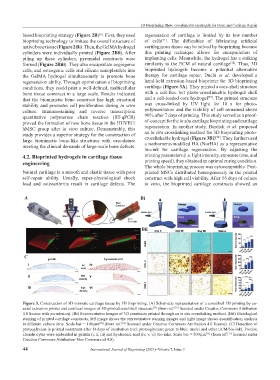
conditions, they could print a well-defined, multicellular cartilage (Figure 3A). They printed a core-shell structure
bone tissue construct in a large scale. Results indicated with a cell-free but photo-crosslinkable hydrogel shell
[73]
that the biomimetic bone construct has high structural and a cell-loaded core hydrogel . The printed structure
stability and promotes cell proliferation during in vitro was cross-linked by UV light for 10 s for photo-
culture. Immunostaining and reverse transcription polymerization and the viability of cell remained above
quantitative polymerase chain reaction (RT-qPCR) 90% after 7 days of printing. This study served as a proof-
proved the formation of new bone tissue in the HUVEC/ of-concept for the in situ cartilage bioprinting and cartilage
hMSC group after in vitro culture. Demonstrably, this regeneration. In another study, Burdick et al. proposed
study provides a superior strategy for the construction of an in situ crosslinking method for 3D bioprinting photo-
[74]
large biomimetic bone-like structures with vasculature crosslinkable hydrogel (Figure 3Bi) . They further used
meeting the clinical demands of large-scale bone defects. a norbornene-modified HA (NorHA) as a representative
bio-ink for cartilage regeneration. By adjusting the
4.2. Bioprinted hydrogels in cartilage tissue printing parameters (i.e. light intensity, exposure time, and
engineering printing speed), they obtained an optimal curing condition.
The whole bioprinting process was cytocompatible. Post-
Natural cartilage is a smooth and elastic tissue with poor printed MSCs distributed homogenously in the printed
self-repair ability. Usually, super-physiological shock construct with high cell viability. After 56 days of culture
load and osteoarthritis result in cartilage defects. The in vitro, the bioprinted cartilage constructs showed an
A Bi
Bii
C
Figure 3. Construction of 3D mimetic cartilage tissue by 3D bioprinting. (A) Schematic representation of a core/shell 3D printing by co-
axial extrusion printer and confocal images of 3D printed core/shell structure (from ref. licensed under Creative Commons Attribution
[73]
[73]
4.0 license with permission). (Bi) Representative images of 3D constructs printed through an in situ crosslinking method. (Bii) Histological
staining of printed cartilage constructs; left image shows the representative staining images and right image shows quantification analysis
in different culture time. Scale bar = 100 μm (from ref. licensed under Creative Commons Attribution 4.0 license). (C) Detection of
[74]
[74]
proteoglycans in printed constructs after 14 days of incubation (red: proteoglycans; green to blue: nuclei and other ECM/bio-ink). Porcine
chondrocytes were embedded in gelatin (i, ii, iii) and hyaluronic acid (iv, v, vi) bio-inks. Scale bar = 500 μm (from ref. licensed under
[75]
[75]
Creative Commons Attribution-Non Commercial 4.0).
44 International Journal of Bioprinting (2021)–Volume 7, Issue 3