Page 96 - IJB-7-4
P. 96
3D Printing Osteochondral Scaffold
A B C
D E F
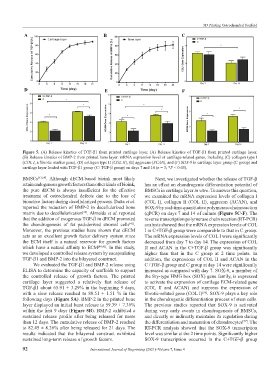
Figure 5. (A) Release kinetics of TGF-β1 from printed cartilage layer. (A) Release kinetics of TGF-β1 from printed cartilage layer.
(B) Release kinetics of BMP-2 from printed bone layer. mRNA expression level of cartilage-related genes, including (C) collagen type I
(COL I, a fibrotic marker gene), (D) collagen type II (COL II), (E) aggrecan (ACAN), and (F) SOX-9 in cartilage layer group (C group) and
cartilage layer loaded with TGF-β1 group (C+TGF-β group) on days 7 and 14 (n = 3; *P < 0.05).
BMSCs [53,54] . Although dECM-based bioink most likely Next, we investigated whether the release of TGF-β
retain endogenous growth factors than other kinds of bioink, has an effect on chondrogenic differentiation potential of
the pure dECM is always insufficient for the effective BMSCs in cartilage layer in vitro. To answer this question,
treatment of osteochondral defects due to the loss of we examined the mRNA expression levels of collagen I
bioactive factors during decellularized process. Datta et al. (COL I), collagen II (COL II), aggrecan (ACAN), and
reported the reduction of BMP-2 in decellularized bone SOX-9 by real-time quantitative polymerase chain reaction
matrix due to decellularization . Almeida et al. reported (qPCR) on days 7 and 14 of culture (Figure 5C-F). The
[55]
that the addition of exogenous TGF-β in dECM promoted reverse transcription polymerase chain reaction (RT-PCR)
the chondrogenesis of fat pad-derived stromal cells . analysis showed that the mRNA expression levels of COL
[41]
Moreover, the previous studies have shown that dECM I in C+TGF-β group were comparable to that in C group.
acts as an excellent growth factor delivery system since The mRNA expression levels of COL I were significantly
the ECM itself is a natural reservoir for growth factors decreased from day 7 to day 14. The expression of COL
which have a natural affinity to ECM [40,41] . In this study, II and ACAN in the C+TGF-β group was significantly
we developed a controlled release system by encapsulating higher than that in the C group at 2 time points. In
TGF-β1 and BMP-2 into the bilayered construct. addition, the expressions of COL II and ACAN in the
We evaluated the TGF-β1 and BMP-2 release using C+TGF-β group and C group at day 14 were significantly
ELISA to determine the capacity of scaffolds to support increased as compared with day 7. SOX-9, a member of
the controlled release of growth factors. The printed the Sry-type HMG box (SOX) gene family, is expressed
cartilage layer suggested a relatively fast release of to activate the expression of cartilage ECM-related gene
TGF-β1 about 65.91 ± 3.29% in the beginning 9 days, (COL II and ACAN) and suppress the expression of
with a slow release reached to 88.51 ± 1.51 % in the fibrotic-related gene (COL I) . SOX-9 plays a key role
[56]
following days (Figure 5A). BMP-2 in the printed bone in the chondrogenic differentiation process of stem cells.
layer displayed an initial burst release to 59.39 ± 7.36% The previous studies reported that SOX-9 is activated
within the first 9 days (Figure 5B). BMP-2 exhibited a during very early events in chondrogenesis of BMSCs,
sustained release profile after being released for more and directly or indirectly maintains its regulation during
than 12 days. The cumulative release of BMP-2 reached the differentiation and maturation of chondrocytes . The
[57]
to 82.45 ± 8.26% after being released for 21 days. The RT-PCR analysis showed that the SOX-9 transcription
results indicated that the bilayered construct exhibited level was similar at the 2 time points. Significantly higher
sustained long-term release of growth factors. SOX-9 transcription occurred in the C+TGF-β group
92 International Journal of Bioprinting (2021)–Volume 7, Issue 4